By PharmaCompass
2024-02-08
Impressions: 2963
The New Year got off to a stable start, with some good news trickling in from clinical trials and Big Pharma announcing a few acquisitions at the annual JP Morgan healthcare meet.
The benchmark index S&P 500 has been on a green streak, growing 15.33 percent since October. In comparison, pharma indices underperformed. The Nasdaq Biotechnology index gained 1 percent in January to 4385.06, mirroring the 1 percent it had gained in December 2023. The SPDR S&P Biotech ETF index lost 1 percent to end January at 87.43 after soaring 18 percent to 89.29 in December. And, the S&P Biotechnology Select Industry Index (SPSIBI) dropped 3 percent in January to 6813.77, after spiking 8 percent in December.
During the month, Sanofi acquired rare disease drugmaker Inhibrx for about US$ 2.2 billion and J&J acquired Ambrx for US$ 2 billion to enter the antibody-drug conjugates (ADC) space. ADCs are biopharmaceuticals designed as a targeted therapy for treating cancer.
Cell therapies have been facing a rough period with narrower funding options that have forced many startups in the biotech sector to shut shop. The field received a further blow when the US Food and Drug Administration (FDA) mandated “black box” warnings — the highest safety warnings — on six major CAR-T cancer therapies over the risk of developing secondary cancer. However, the regulator is also helping out the sector and has finalized guidance for companies and academic researchers working on CAR-T cell therapies.
Access the Pipeline Prospector Dashboard for January 2024 Newsmakers (Free Excel)
Vertex’s non-opioid painkiller scores trial wins; Lilly’s therapy cures deafness
Vertex Pharmaceuticals’ non-opioid painkiller VX-548 met its primary goals in two late-stage trials, marking a major development in a decades-long effort to have an alternative to opioids. The experimental non-opioid painkiller reduced acute, post-surgical pain. It was found to be safe and did the job without the risk of addiction. Vertex plans to file for FDA approval by mid-2024. Analysts estimate VX-548 to bring in peak sales of US$ 5 billion, if approved.
There was good news from clinical trials for those with deafness. In an early-to-mid-stage trial, an 11-year-old boy on Eli Lilly’s investigational gene therapy AK-OTOF, could hear within 30 days of a single administration. Similarly, an experimental gene therapy being developed by a Chinese drugmaker Refreshgene Therapeutics also restored hearing in five out of six children with congenital deafness. Both treatments worked on profound deafness caused by mutations of the OTOF (otoferlin) gene.
Vera Therapeutics (stock up 138 percent), a clinical-stage biotechnology company, announced positive 72-week data from a phase 2b clinical trial of atacicept in participants with IgA nephropathy (IgAN), also known as Berger’s disease. IgAN is a serious and progressive autoimmune disease of the kidney, for which there remains a high unmet medical need. Vera holds an exclusive worldwide license from Merck KGaA for the development and commercialization of atacicept in all indications.
Meanwhile, Regenxbio touted positive interim data from a mid-stage trial for its experimental treatment for wet age-related macular degeneration (wet AMD) that uses a novel delivery, known as suprachoroidal delivery. Regenxbio has developed the drug along with AbbVie.
Access the Pipeline Prospector Dashboard for January 2024 Newsmakers (Free Excel)
FDA finalizes CAR-T guidance; hands Casgevy second nod for genetic blood disorder
FDA has finalized guidance for companies and academic researchers working on CAR-T cell therapies. The guidance enlists the clinical, non-clinical and manufacturing expectations the agency has before a premarket approval (PMA) application is made.
Vertex Pharmaceuticals and CRISPR Therapeutics’ Casgevy has bagged a second FDA approval, this time for transfusion-dependent β-thalassemia, a rare genetic blood disorder that routinely requires blood transfusions.
Across the pond, the European Commission granted marketing authorization to Stada and Alvotech’s Uzpruvo, a biosimilar to Johnson & Johnson’s Stelara (ustekinumab). Biosimilar competition in the €2.5 billion (US$ 2.7 billion) ustekinumab market in the EU could significantly expand patient access to this life-changing biologic therapy within gastroenterology, dermatology, and rheumatology, a press statement said. Meanwhile, Stelara sales grew by nearly 12 percent to about US$ 11 billion in 2023 compared to US$ 9.7 billion the year earlier.
Access the Pipeline Prospector Dashboard for January 2024 Newsmakers (Free Excel)
Sanofi buys Inhibrx for about US$ 2.2 bn; J&J enters ADC space with Ambrx deal
Sanofi has agreed to buy California-based clinical-stage biopharmaceutical company Inhibrx for about US$ 2.2 billion. The acquisition is aimed at acquiring the biopharma’s mid-stage experimental treatment INBRX-101, which will bolster the French drugmaker’s rare genetic disease portfolio. Inhibrx’s other investigational drugs will be spun off into a different company in which Sanofi will hold an 8 percent stake. INBRX-101 is a potential treatment for alpha-1 antitrypsin deficiency, a rare disease that causes the lung tissue to progressively deteriorate.
Meanwhile, Johnson & Johnson announced the acquisition of ADC drug developer Ambrx Biopharma for about US$ 2 billion. This makes J&J the latest drugmaker to bet on ADCs after Pfizer, AbbVie and Merck.
Access the Pipeline Prospector Dashboard for January 2024 Newsmakers (Free Excel)
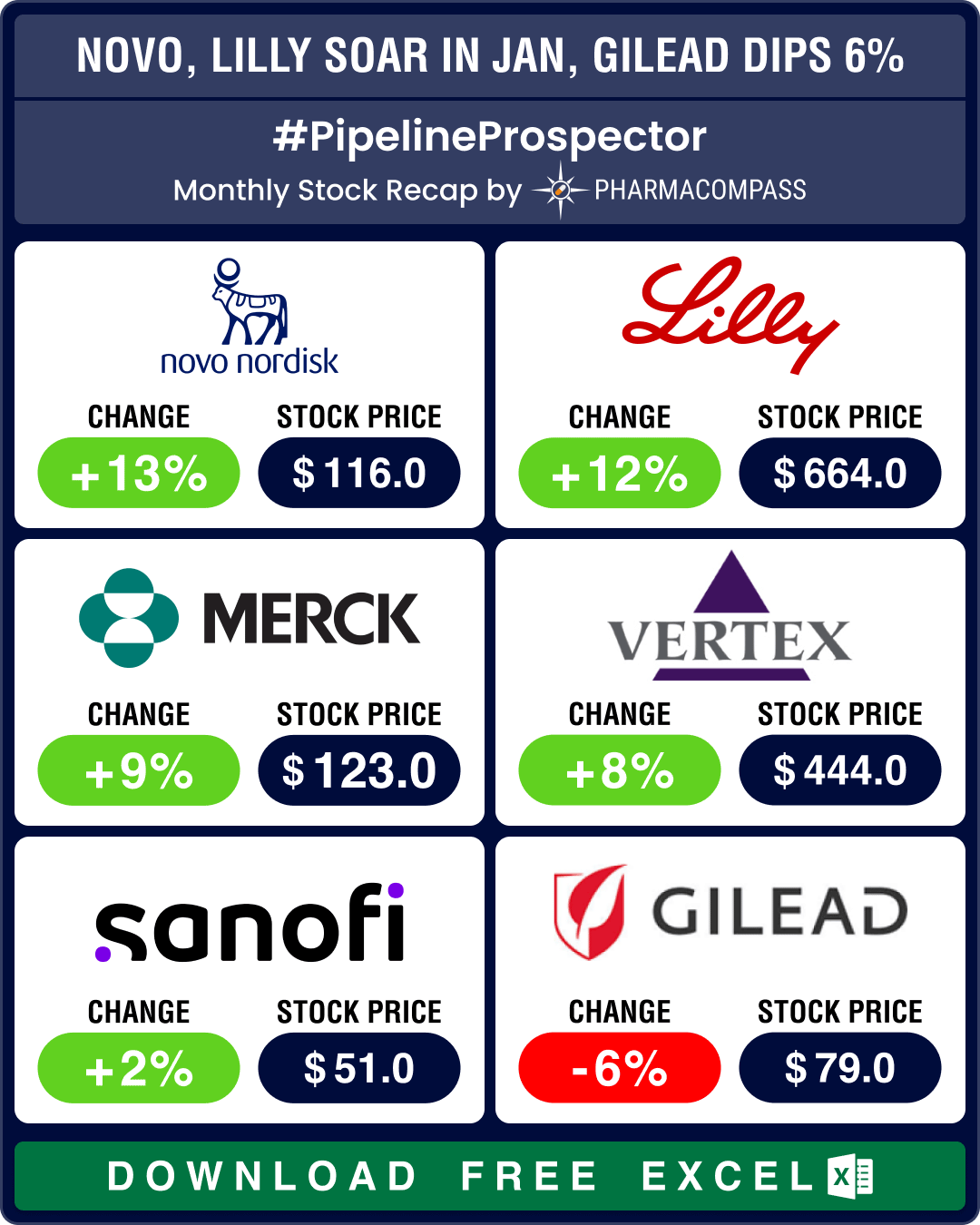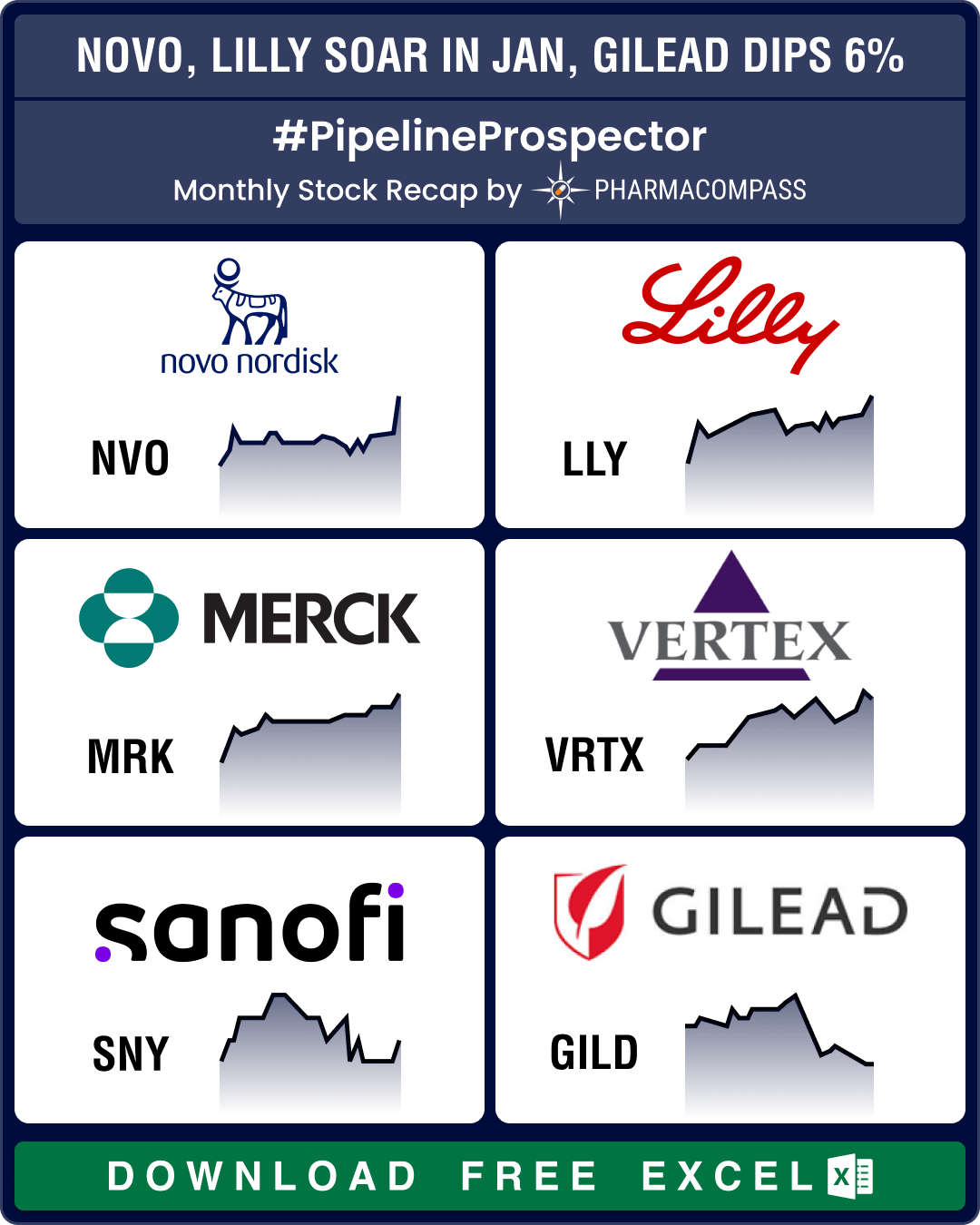
Novo sales soar 36% due to obesity drugs; Pfizer ekes out surprise Q4 2023 profit
The month saw several drugmakers announce financial results for the full year 2023. Novo Nordisk saw sales rise 36 percent at constant exchange rates (CER) to DKK 232.3 billion (US$ 34.36 billion) in 2023 compared to DKK 177 billion (US$ 26.18 billion) in 2022. Ozempic (semaglutide) clocked sales of DKK 95.7 billion (US$ 13.77 billion) compared to DKK 59.6 billion (US$ 8.57 billion), a 66 percent rise. Novo’s stock was up 13 percent in January.Pfizer’s 2023 revenue fell 42 percent year-on-year to US$ 58.5 billion (from the record US$ 100 billion revenue it had posted in 2022) due to a sharp drop in Comirnaty and Paxlovid sales. Yet, Pfizer managed to eke out a profit of US$ 593 million in Q4, when analysts were expecting a US$ 1.1 billion loss. Keytruda raked in US$ 25 billion in 2023, a 19 percent increase over the US$ 21 billion posted in 2022, helping Merck achieve sales of US$ 60.1 billion.
Access the Pipeline Prospector Dashboard for January 2024 Newsmakers (Free Excel)
Our view
The pharma landscape is clearly changing. As financial results for 2023 pour in, we know that our list of top drugmakers is going to look a lot different. We may see further shuffling in 2024, with companies shifting focus to new areas like obesity drugs, ADCs and rare diseases.
The operating environment in 2024 faces “continued risk from geopolitical tension, domestic political uncertainty and heated campaign rhetoric, and increasing attention on regulatory enforcement around the world,” says a PwC report on the sector. Given this scenario, companies that are able to reinvent themself this year with new products and new strategies to fight the changing business and regulatory environment will emerge winners.
Access the Pipeline Prospector Dashboard for January 2024 Newsmakers (Free Excel)
Pharma & Biotech Newsmakers in January 2024
| Company | Country | Currency | Market Cap (Bn) | Change In Market Cap (M) | Stock Price | Change In Price |
|---|
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Pharma & Biotech Newsmakers in January 2024 by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”








