By PharmaCompass
2023-05-11
Impressions: 2593
In March, the collapse of Silicon Valley Bank (SVB) had dragged the biotech indices down. SVB had made considerable investments in the biotech space, and its downfall had left many life sciences and healthcare companies vulnerable.
Within a month of the SVB storm, biotech indices have bounced back. The Nasdaq Biotechnology Index (NBI) gained 1 percent to end April at US$ 4,187.32. It was down 1.3 percent in March. The S&P Biotechnology Select Industry Index (SPSIBI) was up 2 percent at US$ 6,094.82, and the SPDR S&P Biotech ETF (XBI) increased 4 percent to US$ 80.20. Both SPSIBI and XBI had dropped by 8.3 percent and 8.2 percent, respectively, in March.
In April, several drugmakers announced their first quarter (Q1) results. The month also saw three major M&A deals — Merck’s US$ 10.8 billion buyout of Prometheus Biosciences, Astellas Pharma’s US$ 5.9 billion acquisition of Iveric Bio and GSK’s US$ 2 billion deal to buy Bellus Health.
Access the Pipeline Prospector Dashboard for April 2023 Newsmakers (Free Excel)
Pfizer reports 29% drop in Q1 revenues, falls behind GSK in RSV vaccine race
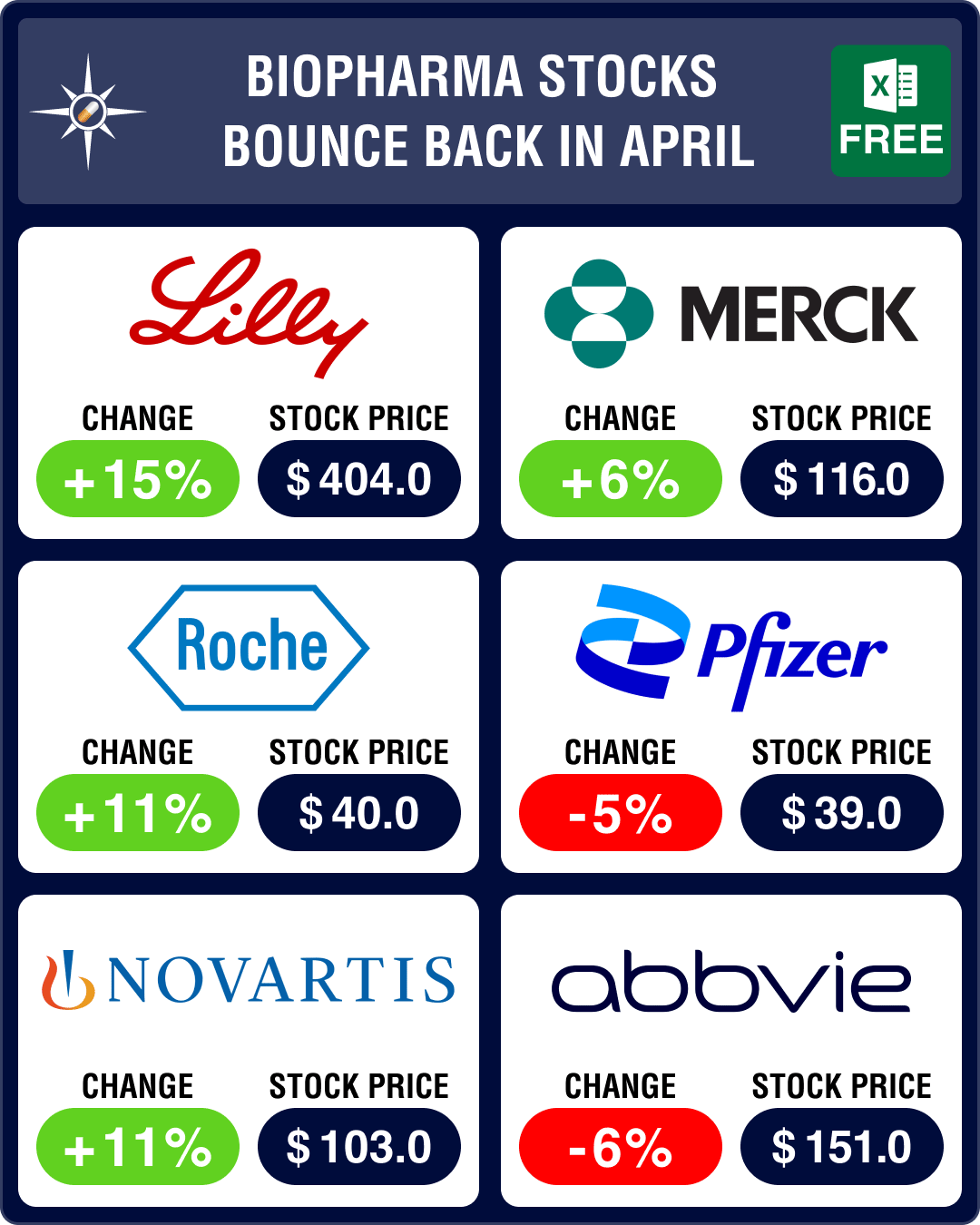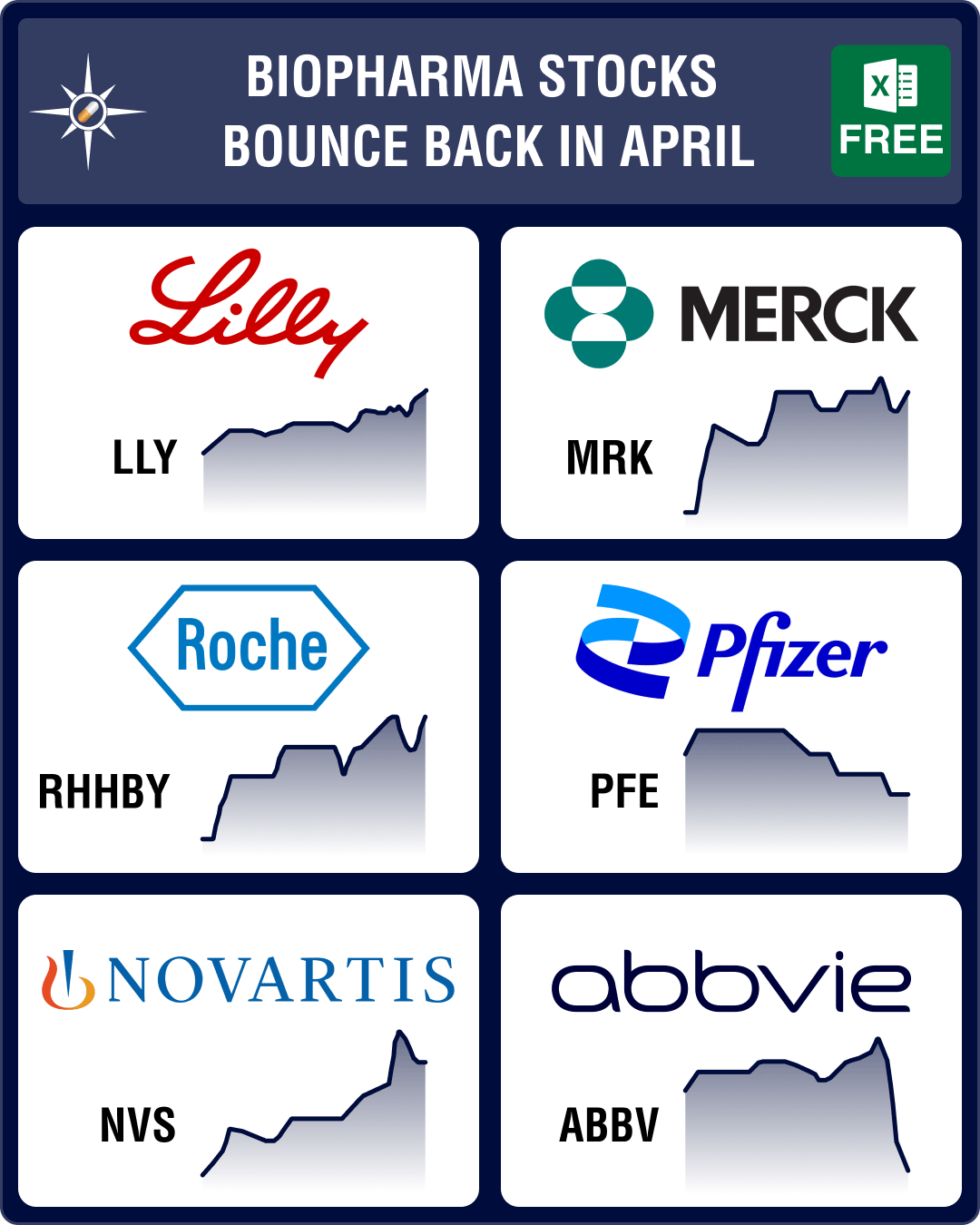
We have seen this happening for many months now, with Pfizer’s stock consistently falling since January. The Q1 results only echoed that sentiment — Pfizer’s revenues fell 29 percent to US$ 18.3 billion due to a 77 percent drop in sales of its Covid-19 vaccine Comirnaty. This led to a further 5 percent drop in the drug behemoth’s stock in April.
What was going right for Pfizer was its experimental respiratory syncytial virus (RSV) vaccine. But GSK raced ahead of Pfizer this month when the US Food and Drug Administration (FDA) approved its RSV vaccine — Arexvy — for adults aged 60 years and older. The Prescription Drug User Fee Act (PDUFA) date for the Pfizer jab for older adults is this month too. GSK also announced the acquisition of Canada-based drug developer Bellus Health (up 114 percent) for US$ 2 billion.
Among other Covid drugmakers, Moderna’s stock also suffered a 17 percent loss last month. What was worse, its experimental messenger RNA-based influenza vaccine failed to demonstrate effectiveness against influenza B. Moderna is planning to go ahead with a phase 3 study of mRNA-4157 (its investigational individualized neo-antigen therapy) and Keytruda combo in patients with adjuvant melanoma this year.
Access the Pipeline Prospector Dashboard for April 2023 Newsmakers (Free Excel)
Wegovy helps boost Novo’s Q1 revenues; Vabysmo brings gains for Roche
In April, both Novo Nordisk and Eli Lilly posted gains in the bourses. Wegovy’s success in the US helped Novo’s obesity care segment notch up a 131 percent increase in Q1 sales. Novo’s diabetes and obesity drug sales rose 33 percent and were at DKK 48.8 billion (US$ 7.11 billion). Overall, the Danish drugmaker posted an impressive 27 percent growth in its Q1 sales — which were at DKK 53.4 billion (US$ 7.7 billion) — while its stock rose 5 percent in April.
Lilly is seeking to compete with Wegovy’s dominance in the obesity market with its own drug, Mounjaro. It recently posted favorable data from a late-stage trial, where Mounjaro achieved 21 percent weight reduction in non-diabetic patients, compared to 15 percent for Wegovy. Lilly is now conducting another Phase 3b trial to test Mounjaro against Wegovy. And in a separate trial, Mounjaro led to a 16 percent weight loss in patients with type 2 diabetes. The drugmaker‘s stock rose by a handsome 15 percent.
Roche Group’s pharmaceutical division posted 9 percent growth in Q1 sales — at CHF 11.7 billion (US$ 12.7 billion) — due to strong demand for newer medicines. Eye diseases drug Vabysmo, approved last year, turned out to be the strongest growth driver, generating CHF 432 million (US$ 486 million) in global sales. Roche expects the drug to generate US$ 2 billion in revenue this year. The Swiss drugmaker also shared post-hoc analyses from four additional phase 3 studies showing Vabysmo to be more effective at drying retinal fluid than Regeneron’s Eylea in Age-related macular degeneration (AMD) and Diabetic Macular Edema (DME). Roche’s stock gained 11 percent.
In addition, FDA approved Roche’s Polivy as part of a five-drug combination for previously untreated diffuse large B-cell lymphoma (DLBCL), the first new treatment in nearly 20 years to significantly improve outcomes in first-line DLBCL. In Q1, Roche reported a decline of 3 percent in group sales due to lower demand for Covid tests.
Access the Pipeline Prospector Dashboard for April 2023 Newsmakers (Free Excel)
Higher sales of key drugs take Novartis’ Q1 sales up 8%; J&J lowers 2023 guidance
Novartis’ Q1 sales grew 8 percent to US$ 12.95 billion, driven by strong performance of its heart failure drug Entresto, cancer meds Pluvicto and Kisqali, and multiple sclerosis drug Kesimpta. Based on higher Q1 sales, the Swiss drugmaker has raised its outlook for 2023.
Novartis and partner BeiGene’s experimental drug tislelizumab showed promising results in treating certain types of gastric cancer in a late-stage trial. The partners hope this data will help make tislelizumab the first-line therapy for patients with advanced forms of gastric cancer. While Novartis’ stock went up 11 percent, BeiGene’s stock gained 17 percent in April.
Though J&J’s Q1 results exceeded analyst expectations, the drugmaker lowered the 2023 guidance for its pharmaceutical business. Leaked data from a phase 3 trial showed that J&J and Legend’s cancer drug Carvykti performed better-than-expected in patients with multiple myeloma. J&J’s stock went up 4 percent. Legend’s stock rose 46 percent.
Among small cap companies, Madrigal’s stock rose 24 percent in April on news of a breakthrough therapy designation for its drug Resmetirom as a treatment for nonalcoholic steatohepatitis.
Access the Pipeline Prospector Dashboard for April 2023 Newsmakers (Free Excel)
Merck acquires Prometheus for US$ 10.8 bn; Astellas buys Iveric Bio for US$ 5.9 bn
April proved to be a good month for both Merck and AstraZeneca, even though the two drugmakers reported a drop in their Q1 revenues due to decreased sales of their Covid-19 products.
While Merck reported a 9 percent drop in Q1 sales (at US$ 14.5 billion), with sales of its Covid-19 antiviral pill Lagevrio (molnupiravir) dropping 88 percent, Astra reported a 4 percent decrease in revenue (at US$ 10.88 billion) in Q1. Stocks of both drugmakers rose by 6 percent.
In early April, FDA had granted accelerated approval to Merck’s Keytruda in combination with Seagen and Astellas Pharma’s Padcev as a first-line treatment for adults with locally advanced or metastatic urothelial carcinoma. Later in the month, Merck announced it is acquiring San Diego-based biotech Prometheus Biosciences for US$ 10.8 billion to strengthen its immunology pipeline. Prometheus’ stock skyrocketed 76 percent on the news.
A combination of AstraZeneca's cancer drugs — Imfinzi and Lynparza — met its main goal in a late-stage trial for patients with advanced epithelial ovarian cancer. Additionally, Astra’s Ultomiris also received a positive recommendation from the European Medicines Agency (EMA) for adult patients with neuromyelitis optica spectrum disorder.
Japanese drugmaker Astellas Pharma announced its biggest deal last month when it acquired New Jersey-based eye drug developer Iveric Bio for US$ 5.9 billion, taking its stock up 9 percent. The deal will give Astellas access to Iveric Bio’s ophthalmology treatments, including its lead drug candidate for geographic atrophy — Zimura. Iveric Bio’s stock gained 22 percent.
Access the Pipeline Prospector Dashboard for April 2023 Newsmakers (Free Excel)
Disappointing Q1 sales drag AbbVie, BMS stocks down
Both AbbVie and Bristol Myers Squibb reported a drop in Q1 sales. AbbVie was hit due to weaker-than-expected performance of its blockbuster drugs Skyrizi and Rinvoq and dwindling sales of Humira (which experienced a sales drop of 25.2 percent in the face of biosimilar competition in the US). Its Q1 sales fell by 9.7 percent to US$ 12.25 billion, and its stock was down 6 percent.
In positive news, FDA expanded the approval of AbbVie’s Qulipta as a preventive treatment for chronic migraine in adults. Rinvoq also received an add-on authorization in the EU as a treatment for moderate-to-severe active Crohn’s disease.
BMS’ Q1 sales dropped 3 percent to US$ 11.3 billion, primarily due to an erosion in Revlimid’s sales in the face of competition from generic drugs. Revlimid’s revenues fell by 37 percent in Q1 compared to the same period last year. BMS’ stock experienced a 4 percent drop.
Access the Pipeline Prospector Dashboard for April 2023 Newsmakers (Free Excel)
Our view
The collapse of three US banks over the last three months has dealt a blow to the US economy. Globally, the business scenario hasn’t improved much, with inflation, high interest rates, ongoing Russia-Ukraine war, supply chain kinks and other uncertainties continuing to pose challenges.
If the biotech indices are able to hold up in these difficult times, it is an indication of strength and resilience within the sector.
Access the Pipeline Prospector Dashboard for April 2023 Newsmakers (Free Excel)
Pharma & Biotech Newsmakers in April 2023
| Company | Country | Currency | Market Cap (Bn) | Change In Market Cap (M) | Stock Price | Change In Price |
|---|
The PharmaCompass Newsletter – Sign Up, Stay Ahead
Feedback, help us to improve. Click here
Image Credit : Pharma & Biotech Newsmakers by PharmaCompass license under CC BY 2.0
“ The article is based on the information available in public and which the author believes to be true. The author is not disseminating any information, which the author believes or knows, is confidential or in conflict with the privacy of any person. The views expressed or information supplied through this article is mere opinion and observation of the author. The author does not intend to defame, insult or, cause loss or damage to anyone, in any manner, through this article.”







