



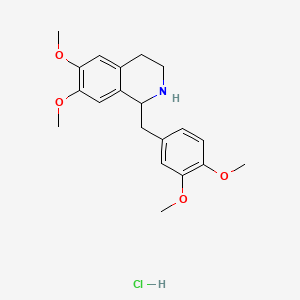

1. Tetrahydropapaverine
2. Tetrahydropapaverine Hydrochloride
1. 6429-04-5
2. Tetrahydropapaverine Hydrochloride
3. Tetrahydropapaverine Hcl
4. Tetrahydroalkali Hydrochloride
5. Dl-norlaudanosine Hydrochloride
6. Norlaudanosine Hydrochloride
7. Schembl6382627
8. Tetrahydropapaverine Hydrochlorid
9. Tetrahydropapaverine (hydrochloride)
10. Mfcd00035267
11. 1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline;hydrochloride
12. 1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline Hydrochloride
13. 1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline Hcl
14. Pubchem14134
15. Mfcd03788189
16. Tetrahydropapaverinehydrochloride
17. Ctk6j7467
18. Norlaudanosine Hcl
19. St50409679
20. Molport-001-770-233
21. Bcp22904
22. Bcp22905
23. Hy-n0137
24. Ccg-49784
25. Nsc118072
26. (r)-tetrahydropapaverine Hydrochloride
27. Akos015900355
28. Ac-8778
29. Ds-2672
30. H29t045
31. Nsc-118072
32. 1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinolinehydrochloride
33. Ak109367
34. Isoquinoline, 1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-, Hydrochloride (1:1)
35. Sy037149
36. Sy258049
37. Db-016699
38. Db-050126
39. Cs-0007858
40. N0918
41. Papaverine,2,3,4-tetrahydro-, Hydrochloride
42. Sw219265-1
43. A806141
44. Q-101021
45. Sr-01000639215-1
46. (r)-1,2,3,4-tetrahydro-6,7-dimethoxy-1-veratrylisoquinoline
47. 1,2,3,4-tetrahydro-1-(3,4-dimethoxybenzyl)-6,7-dimethoxyisoquinoline Hydrochloride
48. 1-(4,5-dimethoxybenzyl)-6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-hydrochloride
49. 1-(4,5-dimethoxybenzyl)6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline-hydrochloride
| Molecular Weight | 379.9 g/mol |
|---|---|
| Molecular Formula | C20H26ClNO4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 6 |
| Exact Mass | 379.1550360 g/mol |
| Monoisotopic Mass | 379.1550360 g/mol |
| Topological Polar Surface Area | 49 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 407 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |




