
Synopsis
Synopsis
0
JDMF
0
KDMF
0
VMF
0
Canada
0
Australia
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
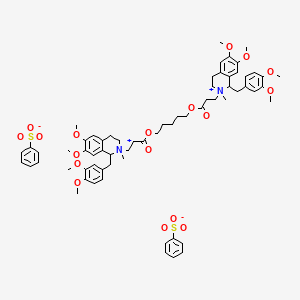

1. 33 A 74
2. A 74, 33
3. Atracurium
4. Atracurium Besilate
5. Atracurium Dibesylate
6. Besilate, Atracurium
7. Bw 33a
8. Bw-33a
9. Bw33a
10. Relatrac
11. Tracrium
1. 64228-81-5
2. Atracurium Besilate
3. Tracrium
4. Atracurium (besylate)
5. Bw-33a
6. Atracurium Dibesylate
7. Atracurii Besilas
8. Besilate D'atracurium
9. Besilato De Atracurio
10. Bw 33a
11. Chebi:2915
12. Benzenesulfonate;5-[3-[1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1h-isoquinolin-2-ium-2-yl]propanoyloxy]pentyl 3-[1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1h-isoquinolin-2-ium-2-yl]propanoate
13. Nsc-760047
14. 2,2'-((pentane-1,5-diylbis(oxy))bis(3-oxopropane-3,1-diyl))bis(1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinolin-2-ium) Benzenesulfonate
15. Atracurium Besilate (inn)
16. Atracurium Besilate [inn]
17. 51w89
18. Tracrium Preservative Free
19. Cis-atracurium Besylate
20. Atracurii Besilas [inn-latin]
21. Hydro-1h-isoquinolin-2-ium-2-yl]propanoate
22. Bw 33 A
23. Besilate D'atracurium [inn-french]
24. Einecs 264-743-4
25. Atracurium Besylate Preservative Free
26. Besilato De Atracurio [inn-spanish]
27. 33 A 74
28. Unii-40ax66p76p
29. Atracurium Besylate [usan:usp]
30. Ncgc00017127-01
31. Tracrium (tn)
32. Benzenesulfonate;5-[3-[(1r,2r)-1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1h-isoquinolin-2-ium-2-yl]propanoyloxy]pentyl 3-[(1r,2r)-1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1h-isoquinolin-2-ium-2-yl]propanoate
33. Benzenesulfonate;5-[3-[1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1h-isoquinolin-2-ium-2-yl]propanoyloxy]pentyl 3-[1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-di
34. Cas-64228-81-5
35. Mfcd00797403
36. Dsstox_cid_2630
37. Atracurium Besylate (usp)
38. Dsstox_rid_76665
39. Dsstox_gsid_22630
40. Schembl41251
41. 2-(2-carboxyethyl)-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium Benzenesulfonate, Pentamethylene Ester
42. Pentamethylen Bis(3-(1,2,3,4-tetrahydro-6,7-dimethoxy-1-(3,4-dimethoxybenzyl)-2-methyl-2-isochinolyl)propionat) Bis(benzolsulfonat)
43. Chembl1200527
44. Dtxsid6022630
45. Hy-b0292a
46. 40ax66p76p
47. Hms1568a11
48. Hms2095a11
49. Hms3651c21
50. Hms3712a11
51. Hms3884g12
52. Pharmakon1600-01505872
53. Bcp22694
54. Bcp22909
55. Benzenesulfonate; 5-[3-[(1r,2r)-1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1h-isoquinolin-2-ium-2-yl]propanoyloxy]pentyl 3-[(1s,2s)-1-[(3,4-dimethoxyphenyl)methyl]-6,7-dimethoxy-2-methyl-3,4-dihydro-1h-isoquinolin-2-ium-2-yl]propan
56. Tox21_110790
57. Nsc760047
58. S1832
59. Akos015895831
60. Ab07163
61. Atracurium Besylate, Mixture Of Isomers
62. Ccg-213566
63. Db00732
64. Nc00429
65. Nsc 760047
66. Ncgc00262598-01
67. Ncgc00262598-02
68. 2,2'-[1,5-pentanediylbis[oxy(3-oxo-3,1-propanediyl)]]bis[1-(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methylisoquinolinium] Dibenzenesulfonate
69. As-14131
70. Isoquinolinium, 2,2'-(1,5-pentanediylbis(oxy(3-oxo-3,1-propanediyl)))bis(1-((3,4-dimethoxyphenyl)methyl)-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-, Dibenzenesulfonate
71. A2566
72. Ft-0657467
73. Ft-0659516
74. Sw197148-4
75. Atracurium Besylate, >=98% (hplc), Powder
76. D00758
77. Q165660
78. Sr-01000781272
79. Q-101018
80. Q-200864
81. Sr-01000781272-3
82. Atracurium Besilate Pound>>bw 33a Pound>>bw33a Pound>>bw-33a
83. Atracurium Besylate, European Pharmacopoeia (ep) Reference Standard
84. Atracurium Besylate, United States Pharmacopeia (usp) Reference Standard
85. Atracurium For Impurity F Identification, European Pharmacopoeia (ep) Reference Standard
86. Atracurium For Peak Identification, European Pharmacopoeia (ep) Reference Standard
87. 2,2'-((pentane-1,5-diylbis(oxy))bis(3-oxopropane-3,1-diyl))bis(1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinolin-2-ium)benzenesulfonate
88. 2,2'-(3,3'-(pentane-1,5-diylbis(oxy))bis(3-oxopropane-3,1-diyl))bis(1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinolinium) Benzenesulfonate
89. 2,2'-[1,5-pentanediylbis[oxy(3-oxo-3,1-propanediyl)]]bis[1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-isoquinolinium Benzenesulfonate
90. 2,2'-{pentane-1,5-diylbis[oxy(3-oxopropane-3,1-diyl)]}bis[1-(3,4-dimethoxybenzyl)-6,7-dimethoxy-2-methyl-1,2,3,4-tetrahydroisoquinolinium] Bisbenzenesulfonate
91. 2,2'-{pentane-1,5-diylbis[oxy(3-oxopropane-3,1-diyl)]}bis[1-{[3,4-bis(methyloxy)phenyl]methyl}-2-methyl-6,7-bis(methyloxy)-1,2,3,4-tetrahydroisoquinolinium] Bisbenzenesulfonate
| Molecular Weight | 1243.5 g/mol |
|---|---|
| Molecular Formula | C65H82N2O18S2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 18 |
| Rotatable Bond Count | 26 |
| Exact Mass | 1242.50040612 g/mol |
| Monoisotopic Mass | 1242.50040612 g/mol |
| Topological Polar Surface Area | 258 Ų |
| Heavy Atom Count | 87 |
| Formal Charge | 0 |
| Complexity | 1560 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 4 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
| 1 of 4 | |
|---|---|
| Drug Name | Atracurium besylate |
| Drug Label | Atracurium besylate is an intermediate-duration, nondepolarizing, skeletal muscle relaxant for intravenous administration. Atracurium besylate is designated as 2-(2-Carboxyethyl)-1, 2, 3, 4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium b... |
| Active Ingredient | Atracurium besylate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Hospira; Sagent Pharms; Eurohlth Intl |
| 2 of 4 | |
|---|---|
| Drug Name | Atracurium besylate preservative free |
| Active Ingredient | Atracurium besylate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Hospira; Sagent Pharms; Eurohlth Intl |
| 3 of 4 | |
|---|---|
| Drug Name | Atracurium besylate |
| Drug Label | Atracurium besylate is an intermediate-duration, nondepolarizing, skeletal muscle relaxant for intravenous administration. Atracurium besylate is designated as 2-(2-Carboxyethyl)-1, 2, 3, 4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium b... |
| Active Ingredient | Atracurium besylate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Hospira; Sagent Pharms; Eurohlth Intl |
| 4 of 4 | |
|---|---|
| Drug Name | Atracurium besylate preservative free |
| Active Ingredient | Atracurium besylate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 10mg/ml |
| Market Status | Prescription |
| Company | Hospira; Sagent Pharms; Eurohlth Intl |
For use, as an adjunct to general anesthesia, to facilitate endotracheal intubation and to provide skeletal muscle relaxation during surgery or mechanical ventilation.
Atracurium is a nondepolarizing skeletal muscle relaxant. Atracurium can be used most advantageously if muscle twitch response to peripheral nerve stimulation is monitored to assess degree of muscle relaxation. The duration of neuromuscular block produced by Atracurium is approximately one third to one half the duration of block by d-tubocurarine, metocurine, and pancuronium at initially equipotent doses. As with other nondepolarizing neuromuscular blockers, the time to onset of paralysis decreases and the duration of maximum effect increases with increasing doses of Atracurium. Repeated administration of maintenance doses of Atracurium has no cumulative effect on the duration of neuromuscular block if recovery is allowed to begin prior to repeat dosing. Moreover, the time needed to recover from repeat doses does not change with additional doses. Repeat doses can therefore be administered at relatively regular intervals with predictable results.
Neuromuscular Nondepolarizing Agents
Drugs that interrupt transmission at the skeletal neuromuscular junction without causing depolarization of the motor end plate. They prevent acetylcholine from triggering muscle contraction and are used as muscle relaxants during electroshock treatments, in convulsive states, and as anesthesia adjuvants. (See all compounds classified as Neuromuscular Nondepolarizing Agents.)
Nicotinic Antagonists
Drugs that bind to nicotinic cholinergic receptors (RECEPTORS, NICOTINIC) and block the actions of acetylcholine or cholinergic agonists. Nicotinic antagonists block synaptic transmission at autonomic ganglia, the skeletal neuromuscular junction, and at central nervous system nicotinic synapses. (See all compounds classified as Nicotinic Antagonists.)
The elimination half-life is approximately 20 minutes.
Atracurium antagonizes the neurotransmitter action of acetylcholine by binding competitively with cholinergic receptor sites on the motor end-plate. This antagonism is inhibited, and neuromuscular block reversed, by acetylcholinesterase inhibitors such as neostigmine, edrophonium, and pyridostigmine.
 Synnat Pharma is one of the leading active pharmaceutical ingredients and intermediates manufacturers.
Synnat Pharma is one of the leading active pharmaceutical ingredients and intermediates manufacturers.

Date of Issue : 2024-05-22
Valid Till : 2027-02-07
Written Confirmation Number : WC-0301
Address of the Firm :
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
Shanghai Minbiotech is the leading producer of biopharmaceuticals and a variety of high-end generic & innovative drugs.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.

GDUFA
DMF Review : Reviewed
Rev. Date : 2013-08-28
Pay. Date : 2013-08-16
DMF Number : 11742
Submission : 1995-11-14
Status : Active
Type : II

Certificate Number : CEP 2006-044 - Rev 07
Issue Date : 2025-06-27
Type : Chemical
Substance Number : 1970
Status : Valid

| Available Reg Filing : CA, ASMF |


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21189
Submission : 2007-12-03
Status : Active
Type : II

Certificate Number : CEP 2008-272 - Rev 01
Issue Date : 2024-11-28
Type : Chemical
Substance Number : 1970
Status : Valid
Date of Issue : 2022-04-08
Valid Till : 2025-04-03
Written Confirmation Number : WC-0427
Address of the Firm :



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 29636
Submission : 2015-08-28
Status : Active
Type : II



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38238
Submission : 2023-03-31
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 18444
Submission : 2005-06-21
Status : Inactive
Type : II

Certificate Number : R1-CEP 2008-013 - Rev 00
Issue Date : 2014-09-29
Type : Chemical
Substance Number : 1970
Status : Valid

| Available Reg Filing : ASMF |


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 11144
Submission : 1994-10-21
Status : Inactive
Type : II


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
 TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
TAPI, a leading global supplier of APIs, provides over 350 products and customized CDMO solutions for every stage of development.
Certificate Number : CEP 2006-044 - Rev 07
Status : Valid
Issue Date : 2025-06-27
Type : Chemical
Substance Number : 1970
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2013-019 - Rev 00
Status : Valid
Issue Date : 2020-02-26
Type : Chemical
Substance Number : 1970

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Atracurium Besilate, Manufacturing Code: 55
Certificate Number : CEP 2020-436 - Rev 03
Status : Valid
Issue Date : 2025-12-22
Type : Chemical
Substance Number : 1970

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2008-013 - Rev 00
Status : Valid
Issue Date : 2014-09-29
Type : Chemical
Substance Number : 1970

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : R1-CEP 2006-155 - Rev 00
Status : Valid
Issue Date : 2013-01-31
Type : Chemical
Substance Number : 1970

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.

Certificate Number : CEP 2008-272 - Rev 01
Status : Valid
Issue Date : 2024-11-28
Type : Chemical
Substance Number : 1970

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] 
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
CAS Number : 64228-78-0
End Use API : Atracurium Besylate
About The Company : Synnat Pharma is a fast-growing pharmaceutical company focused on the development, identification, production, and distribution of phytochemicals and botanical ...
CAS Number : 6429-04-5
End Use API : Atracurium Besylate
About The Company : Suanfarma is a B2B life sciences company focused on developing, manufacturing, and distributing high-quality ingredients for the pharmaceutical industry in an i...
CAS Number : 54417-53-7
End Use API : Atracurium Besylate
About The Company : Suanfarma is a B2B life sciences company focused on developing, manufacturing, and distributing high-quality ingredients for the pharmaceutical industry in an i...
CAS Number : 111-29-5
End Use API : Atracurium Besylate
About The Company : Suanfarma is a B2B life sciences company focused on developing, manufacturing, and distributing high-quality ingredients for the pharmaceutical industry in an i...
R-Tetrahydropapaverine N-acetyl-L-leucinate
CAS Number : 141109-12-8
End Use API : Atracurium Besylate
About The Company : Shanghai Minbiotech is specializing in the R&D and production of advanced pharmaceutical intermediates and biological APIs. There are more than 1000 square mete...
CAS Number : 54417-53-7
End Use API : Atracurium Besylate
About The Company : Shanghai Minbiotech is specializing in the R&D and production of advanced pharmaceutical intermediates and biological APIs. There are more than 1000 square mete...
3,4-Dimethoxyphenyl acetonitrile (Homoveratryl nit...
CAS Number : 93-17-4
End Use API : Atracurium Besylate
About The Company : Established in 2003 with small pilot plant and came in to commercial production in 2013 in the name of Allchem Laboratories, it is an independent privately owne...

3,4-Dimethoxyphenethylamine (Homoveratryl Amine)
CAS Number : 120-20-7
End Use API : Atracurium Besylate
About The Company : Established in 2003 with small pilot plant and came in to commercial production in 2013 in the name of Allchem Laboratories, it is an independent privately owne...

3,4-Dimethoxyphenylacetic Acid (Homoveratrumic aci...
CAS Number : 93-40-3
End Use API : Atracurium Besylate
About The Company : Established in 2003 with small pilot plant and came in to commercial production in 2013 in the name of Allchem Laboratories, it is an independent privately owne...

CAS Number : 6429-04-5
End Use API : Atracurium Besylate
About The Company : Aventus Labs is a forward-thinking pharmaceutical company committed to developing and delivering high-quality, effective healthcare solutions. With a focus on r...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Tracrium
Dosage Form : Injectable And Infusion Solution
Dosage Strength : 10MG
Packaging :
Approval Date : 01-09-1987
Application Number : 57335
Regulatory Info : Authorized
Registration Country : Spain
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Withdrawn
Registration Country : Malta
Brand Name : Tracrium
Dosage Form : Solution For Injection And Infusion
Dosage Strength : 10MG/ML
Packaging :
Approval Date : 2005-11-25
Application Number :
Regulatory Info : Withdrawn
Registration Country : Malta
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Tracrium Injection 5ml
Dosage Form : INJ
Dosage Strength : 50mg/5ml
Packaging : 5X5mg/5ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Originator
Registration Country : South Africa
Brand Name : Tracrium Injection 2,5 Ml
Dosage Form : INJ
Dosage Strength : 25mg/2.5ml
Packaging : 2.5X5mg/2.5ml
Approval Date :
Application Number :
Regulatory Info : Originator
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : TRACRIUM PRESERVATIVE FREE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 10MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 1983-11-23
Application Number : 18831
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : TRACRIUM
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 10MG/ML **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 1985-06-20
Application Number : 18831
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : ATRACURIUM BESYLATE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 10MG/ML
Packaging :
Approval Date : 1996-12-23
Application Number : 74632
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : ATRACURIUM BESYLATE PRESERVATIVE FREE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 10MG/ML
Packaging :
Approval Date : 1996-12-23
Application Number : 74633
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : ATRACURIUM BESYLATE PRESERVATIVE FREE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 10MG/ML
Packaging :
Approval Date : 1997-03-25
Application Number : 74639
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : ATRACURIUM BESYLATE
Dosage Form : INJECTABLE;INJECTION
Dosage Strength : 10MG/ML
Packaging :
Approval Date : 1997-03-28
Application Number : 74740
Regulatory Info : DISCN
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Tracrium
Dosage Form : Injectable And Infusion Solution
Dosage Strength : 10MG
Packaging :
Approval Date : 01-09-1987
Application Number : 57335
Regulatory Info : Authorized
Registration Country : Spain
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Withdrawn
Registration Country : Malta
Brand Name : Tracrium
Dosage Form : Solution For Injection And Infusion
Dosage Strength : 10MG/ML
Packaging :
Approval Date : 2005-11-25
Application Number :
Regulatory Info : Withdrawn
Registration Country : Malta
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Deregistered
Registration Country : Sweden
Brand Name : Tracrium (Without Preservatives)
Dosage Form : Injectable Solution
Dosage Strength : 10mg/ml
Packaging :
Approval Date : 31/01/1986
Application Number : 19860131000021
Regulatory Info : Deregistered
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Atracurium
Dosage Form : Atracurium 50Mg 5Ml 5 Units Parenteral Use
Dosage Strength : 5 EV vials 50 mg 5 ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Approved
Registration Country : Sweden
Brand Name : Atracurium Hameln
Dosage Form : Solution For Injection/Infusion
Dosage Strength : 10mg/ml
Packaging :
Approval Date : 19/04/2002
Application Number : 20020419000044
Regulatory Info : Approved
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Moldova
Brand Name : Atracurium Kalceks
Dosage Form : Injectable Solution
Dosage Strength : 10mg/ml
Packaging :
Approval Date : 26-05-2023
Application Number :
Regulatory Info :
Registration Country : Moldova

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Prescription
Registration Country : Estonia
Brand Name : Atracurium Besilate Kalceks
Dosage Form : Solution For Injection/Infusion
Dosage Strength : 10mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info : Prescription
Registration Country : Estonia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Authorized
Registration Country : Spain
Brand Name : Besilato De Atracurio Kalceks
Dosage Form : Injectable And Infusion Solution
Dosage Strength : 10MG
Packaging :
Approval Date : 05-04-2022
Application Number : 86777
Regulatory Info : Authorized
Registration Country : Spain

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : Italy
Brand Name : Acurmil
Dosage Form : Injectable
Dosage Strength : 50MG/5ML
Packaging :
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Withdrawn
Registration Country : Malta
Brand Name : Atracurium Hospira
Dosage Form : Solution For Injection
Dosage Strength : 10MG
Packaging :
Approval Date : 2019-06-25
Application Number :
Regulatory Info : Withdrawn
Registration Country : Malta

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
94
PharmaCompass offers a list of Atracurium Besylate API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Atracurium Besylate manufacturer or Atracurium Besylate supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Atracurium Besylate manufacturer or Atracurium Besylate supplier.
PharmaCompass also assists you with knowing the Atracurium Besylate API Price utilized in the formulation of products. Atracurium Besylate API Price is not always fixed or binding as the Atracurium Besylate Price is obtained through a variety of data sources. The Atracurium Besylate Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Atracurium Besylate manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Atracurium Besylate, including repackagers and relabelers. The FDA regulates Atracurium Besylate manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Atracurium Besylate API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Atracurium Besylate manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Atracurium Besylate supplier is an individual or a company that provides Atracurium Besylate active pharmaceutical ingredient (API) or Atracurium Besylate finished formulations upon request. The Atracurium Besylate suppliers may include Atracurium Besylate API manufacturers, exporters, distributors and traders.
click here to find a list of Atracurium Besylate suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Atracurium Besylate DMF (Drug Master File) is a document detailing the whole manufacturing process of Atracurium Besylate active pharmaceutical ingredient (API) in detail. Different forms of Atracurium Besylate DMFs exist exist since differing nations have different regulations, such as Atracurium Besylate USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Atracurium Besylate DMF submitted to regulatory agencies in the US is known as a USDMF. Atracurium Besylate USDMF includes data on Atracurium Besylate's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Atracurium Besylate USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Atracurium Besylate suppliers with USDMF on PharmaCompass.
A Atracurium Besylate CEP of the European Pharmacopoeia monograph is often referred to as a Atracurium Besylate Certificate of Suitability (COS). The purpose of a Atracurium Besylate CEP is to show that the European Pharmacopoeia monograph adequately controls the purity of Atracurium Besylate EP produced by a given manufacturer. Suppliers of raw materials can prove the suitability of Atracurium Besylate to their clients by showing that a Atracurium Besylate CEP has been issued for it. The manufacturer submits a Atracurium Besylate CEP (COS) as part of the market authorization procedure, and it takes on the role of a Atracurium Besylate CEP holder for the record. Additionally, the data presented in the Atracurium Besylate CEP (COS) is managed confidentially and offers a centralized system acknowledged by numerous nations, exactly like the Atracurium Besylate DMF.
A Atracurium Besylate CEP (COS) is recognised by all 36 nations that make up the European Pharmacopoeia Convention. Atracurium Besylate CEPs may be accepted in nations that are not members of the Ph. Eur. at the discretion of the authorities there.
click here to find a list of Atracurium Besylate suppliers with CEP (COS) on PharmaCompass.
A Atracurium Besylate written confirmation (Atracurium Besylate WC) is an official document issued by a regulatory agency to a Atracurium Besylate manufacturer, verifying that the manufacturing facility of a Atracurium Besylate active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Atracurium Besylate APIs or Atracurium Besylate finished pharmaceutical products to another nation, regulatory agencies frequently require a Atracurium Besylate WC (written confirmation) as part of the regulatory process.
click here to find a list of Atracurium Besylate suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Atracurium Besylate as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Atracurium Besylate API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Atracurium Besylate as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Atracurium Besylate and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Atracurium Besylate NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Atracurium Besylate suppliers with NDC on PharmaCompass.
Atracurium Besylate Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Atracurium Besylate GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Atracurium Besylate GMP manufacturer or Atracurium Besylate GMP API supplier for your needs.
A Atracurium Besylate CoA (Certificate of Analysis) is a formal document that attests to Atracurium Besylate's compliance with Atracurium Besylate specifications and serves as a tool for batch-level quality control.
Atracurium Besylate CoA mostly includes findings from lab analyses of a specific batch. For each Atracurium Besylate CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Atracurium Besylate may be tested according to a variety of international standards, such as European Pharmacopoeia (Atracurium Besylate EP), Atracurium Besylate JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Atracurium Besylate USP).

