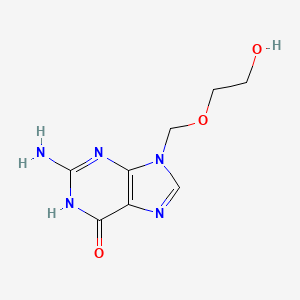

1. 9-((2-hydroxyethoxy)methyl)guanine
2. Aci Sanorania
3. Aci-sanorania
4. Acic
5. Aciclobeta
6. Aciclostad
7. Aciclovir
8. Aciclovir Alonga
9. Aciclovir Sanorania
10. Aciclovir Von Ct
11. Aciclovir-sanorania
12. Acifur
13. Acipen Solutab
14. Acivir
15. Activir
16. Acyclo V
17. Acyclo-v
18. Acycloguanosine
19. Acyclovir Sodium
20. Alonga, Aciclovir
21. Antiherpes Creme
22. Avirax
23. Cicloferon
24. Clonorax
25. Cusiviral
26. Genvir
27. Herpetad
28. Herpofug
29. Herpotern
30. Herpoviric
31. Isavir
32. Laciken
33. Mapox
34. Maynar
35. Milavir
36. Opthavir
37. Sodium, Acyclovir
38. Solutab, Acipen
39. Supraviran
40. Viclovir
41. Vipral
42. Virax Puren
43. Virax-puren
44. Viraxpuren
45. Virherpes
46. Virmen
47. Virolex
48. Virupos
49. Virzin
50. Wellcome 248u
51. Wellcome-248u
52. Wellcome248u
53. Zoliparin
54. Zovirax
55. Zyclir
1. Aciclovir
2. 59277-89-3
3. Acycloguanosine
4. Virorax
5. Aciclovirum
6. Zovir
7. Sitavig
8. Gerpevir
9. Hascovir
10. Aciclovirum [latin]
11. Acicloftal
12. Cargosil
13. Novirus
14. W-248-u
15. Aciclovirum [inn-latin]
16. Bw-248u
17. Drg-0008
18. Ccris 1953
19. Chebi:2453
20. Nsc 645011
21. Hsdb 6511
22. Unii-x4hes1o11f
23. Einecs 261-685-1
24. X4hes1o11f
25. Nsc-645011
26. Nsc-758477
27. Avaclyr
28. Dtxsid1022556
29. Acyclovir [usan:usp]
30. Nsc645011
31. Dtxcid102556
32. Aciclovirum (latin)
33. Acyclovir (usan:usp)
34. Aciclovir (iarc)
35. Aciclovir [iarc]
36. Aciclovirum (inn-latin)
37. Aciclovir (mart.)
38. Aciclovir [mart.]
39. Acyclovir (usp-rs)
40. Acyclovir [usp-rs]
41. Aciclovir (ep Monograph)
42. Aciclovir [ep Monograph]
43. Acyclovir (usp Monograph)
44. Acyclovir [usp Monograph]
45. Valaciclovir Hydrochloride Impurity B (ep Impurity)
46. Valaciclovir Hydrochloride Impurity B [ep Impurity]
47. Aclovir
48. Valaciclovir Hydrochloride Hydrate Impurity B (ep Impurity)
49. Valaciclovir Hydrochloride Hydrate Impurity B [ep Impurity]
50. D06bb03
51. J05ab01
52. S01ad03
53. Nsc335752
54. 261-685-1
55. Acv
56. Zovirax
57. Vipral
58. 9-[(2-hydroxyethoxy)methyl]guanine
59. Wellcome-248u
60. 2-amino-9-((2-hydroxyethoxy)methyl)-1h-purin-6(9h)-one
61. 9-(2-hydroxyethoxy)methylguanine
62. Acyclovir-side Chain-2-3h
63. 9-hyroxyethoxymethylguanine
64. 9-((2-hydroxyethoxy)methyl)guanine
65. Mfcd00057880
66. 2-amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-6h-purin-6-one
67. 141294-79-3
68. Aciclovir [inn]
69. 2-amino-9-(2-hydroxyethoxymethyl)-3h-purin-6-one
70. Acyclovir (aciclovir)
71. 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6h-purin-6-one
72. 2-amino-9-[(2-hydroxyethoxy)methyl]-1,9-dihydro-6h-purin-6-one
73. 6h-purin-6-one, 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-
74. Acyclovir-sidechain-2-3h
75. Zovirax (tn)
76. 9-[(2-hydroxyethoxy)-methyl]guanine
77. 2-amino-9-(2-hydroxyethoxymethyl)-1h-purin-6-one
78. Mls000069633
79. Activir
80. 9-(2-hydroxyethoxymethyl)guanine
81. 6h-purin-6-one, 2-amino-1,9-dihydro-9-((2-hydroxyethoxy)methyl)-
82. Ac2
83. Acyclovir [usan]
84. Ncgc00015061-02
85. Aciclovier
86. Smr000058225
87. Genvir
88. Maynar
89. Zyclir
90. 2-amino-9-[(2-hydroxyethoxy)methyl]-6,9-dihydro-3h-purin-6-one
91. Cas-59277-89-3
92. 6h-purin-6-one, 1,9-dihydro-2-amino-9-((2-hydroxyethoxy)methyl)-
93. 2-amino-9-(2-hydroxyethoxymethyl)purin-6-ol
94. 2-amino-9-((2-hydroxyethoxy)methyl)-3h-purin-6(9h)-one
95. Viropump
96. Acyclofoam
97. 2-amino-9-((2-hydroxyethoxy)methyl)-1,9-dihydro-6h-purin-6-one
98. 2-amino-9-[(2-hydroxyethoxy)methyl]-6,9-dihydro-1h-purin-6-one
99. Acic
100. C8h11n5o3
101. 2-amino-9-{[(2-hydroxyethyl)oxy]methyl}-1,9-dihydro-6h-purin-6-one
102. Acyclo-v
103. Acyclovir Lauriad
104. Acyclovir (usp)
105. 88713-38-6
106. Sr-01000075540
107. Acv & Pluronic F-68
108. B-lf& Acv
109. Acyclovir & Pluronic F-68
110. Cyclovir
111. Poviral
112. Sitavir
113. Prestwick_6
114. 1pwy
115. Bw-248-u
116. Sitavig (tn)
117. Acyclovir (standard)
118. Spectrum_001739
119. Aciclovir [jan]
120. Acyclovir [mi]
121. Acyclovir [hsdb]
122. Opera_id_1674
123. Prestwick0_000086
124. Prestwick1_000086
125. Prestwick2_000086
126. Prestwick3_000086
127. Spectrum2_001563
128. Spectrum3_001874
129. Spectrum4_000225
130. Spectrum5_001093
131. Acyclovir [vandf]
132. Lopac-a-4669
133. Aciclovir (jp18/inn)
134. Chembl184
135. A 4669
136. Aciclovir [who-dd]
137. Aciclovir [who-ip]
138. Schembl3175
139. Lopac0_000037
140. Aciclovirum [who-ip]
141. Bspbio_000012
142. Bspbio_003348
143. Kbiogr_000889
144. Kbioss_002219
145. Bidd:gt0646
146. Divk1c_000185
147. Spectrum1503603
148. Spbio_001466
149. Spbio_001951
150. 2-amino-9-[(2-hydroxyethoxy)methyl]hydropurin-6-one
151. Bpbio1_000014
152. Gtpl4829
153. Schembl9828560
154. 9(2-hydroxyethoxymethyl)guanine
155. Acyclovir [orange Book]
156. Hms500j07
157. Kbio1_000185
158. Kbio2_002219
159. Kbio2_004787
160. Kbio2_007355
161. Kbio3_002850
162. Ninds_000185
163. Glxc-04946
164. Hms1568a14
165. Hms1922e08
166. Hms2090g09
167. Hms2095a14
168. Hms2234k21
169. Hms3259n10
170. Hms3260g15
171. Hms3269m15
172. Hms3372k02
173. Hms3413d21
174. Hms3655c14
175. Hms3677d21
176. Hms3712a14
177. Pharmakon1600-01503603
178. 2-amino-9-[(2-hydroxyethoxy)methyl]-3,9-dihydro-6h-purin-6-one
179. Bcp11036
180. 9-(2-hydroxyethoxy Methyl) Guanine
181. Tox21_110075
182. Tox21_500037
183. 69657-51-8 (na Salt)
184. Bbl009642
185. Bdbm50021776
186. Bdbm50103518
187. Ccg-39909
188. Hy-17422r
189. Nsc758477
190. Nsc780378
191. S1807
192. Stk796771
193. Stl257059
194. Stl301862
195. Akos000656213
196. Akos015995680
197. Akos022135433
198. Tox21_110075_1
199. Ac-8068
200. Cs-1353
201. Db00787
202. Ia58279
203. Ks-1027
204. Lp00037
205. Nc00717
206. Nsc-780378
207. Sdccgsbi-0050026.p003
208. Bovine Lactoferrin & 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6h-purin-6-one
209. Idi1_000185
210. Smp1_000007
211. Ncgc00015061-01
212. Ncgc00015061-03
213. Ncgc00015061-04
214. Ncgc00015061-05
215. Ncgc00015061-06
216. Ncgc00015061-07
217. Ncgc00015061-08
218. Ncgc00015061-09
219. Ncgc00015061-10
220. Ncgc00015061-12
221. Ncgc00015061-13
222. Ncgc00015061-28
223. Ncgc00015061-29
224. Ncgc00022426-03
225. Ncgc00093555-01
226. Ncgc00093555-02
227. Ncgc00093555-03
228. Ncgc00093555-04
229. Ncgc00167756-01
230. Ncgc00167756-02
231. Ncgc00260722-01
232. Ncgc00381719-03
233. Hy-17422
234. Sy051130
235. Acycloguanosine, >=99% (hplc), Powder
236. Aciclovir 1.0 Mg/ml In Dimethyl Sulfoxide
237. A1915
238. Eu-0100037
239. Ns00009000
240. Sw196324-3
241. C06810
242. D00222
243. En300-123010
244. A832236
245. Q147101
246. Sr-01000075540-1
247. Sr-01000075540-3
248. Sr-01000075540-5
249. 2-amino-9-[(2-hydroxyethoxy)methyl]-9h-purin-6-ol
250. 2-azanyl-9-(2-hydroxyethyloxymethyl)-3h-purin-6-one
251. Brd-k32318651-001-17-9
252. Brd-k32318651-001-21-1
253. Brd-k32318651-001-22-9
254. Brd-k32318651-001-23-7
255. Brd-k32318651-001-24-5
256. Aciclovir, British Pharmacopoeia (bp) Reference Standard
257. F2173-0946
258. Z1546610471
259. Aciclovir, European Pharmacopoeia (ep) Reference Standard
260. 2-amino-9-(2-hydroxy-ethoxymethyl)-5,9-dihydro-purin-6-one
261. Acyclovir, United States Pharmacopeia (usp) Reference Standard
262. 2-amino-9-(2-hydroxy-ethoxymethyl)-5,9-dihydro-purin-6-one (acv)
263. 2-amino-9-[(2-hydroxyethoxy)methyl]-1,9-dihydro-6h-purin-6-one #
264. Acv; Acycloguanosine; Acyclovir; Nsc 645011; Nsc-645011; Nsc645011
265. Acyclovir, Pharmaceutical Secondary Standard; Certified Reference Material
266. Aciclovir For Peak Identification 1, European Pharmacopoeia (ep) Reference Standard
267. Aciclovir For Peak Identification 2, European Pharmacopoeia (ep) Reference Standard
268. Aciclovir For System Suitability, European Pharmacopoeia (ep) Reference Standard
269. 2-amino-1 ,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6h-purin-6-one;valaciclovir Hydrochloride Anhydrous Impurity B;acycloguanosine;9-(2- Hydroxyethoxymethyl)guanine
270. Inchi=1/c8h11n5o3/c9-8-11-6-5(7(15)12-8)10-3-13(6)4-16-2-1-14/h3,14h,1-2,4h2,(h3,9,11,12,15
| Molecular Weight | 225.20 g/mol |
|---|---|
| Molecular Formula | C8H11N5O3 |
| XLogP3 | -1.9 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 4 |
| Exact Mass | Da |
| Monoisotopic Mass | Da |
| Topological Polar Surface Area | 115 |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 308 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antiviral Agents
National Library of Medicine's Medical Subject Headings. Acyclovir. Online file (MeSH, 2014). Available from, as of November 19, 2013: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
IV acyclovir sodium is used for the treatment of initial and recurrent mucocutaneous herpes simplex virus (HSV-1 and HSV-2) infections and the treatment of varicella-zoster infections in immunocompromised adults and children; for the treatment of severe first episodes of genital herpes infections in immunocompetent individuals; and for the treatment of HSV encephalitis and neonatal HSV infections.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 766
Acyclovir is used orally for the treatment of initial and recurrent episodes of genital herpes; for the acute treatment of herpes zoster (shingles, zoster) in immunocompetent individuals; and for the treatment of varicella (chickenpox) in immunocompetent individuals.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 766
Oral acyclovir is indicated in the treatment of initial episodes of genital herpes infection in immunocompetent and immunocompromised patients. Parenteral acyclovir is indicated in the treatment of severe initial episodes of genital herpes infection in immunocompetent patients and in patients who are unable to take (or absorb) oral acyclovir. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 26
For more Therapeutic Uses (Complete) data for ACYCLOVIR (15 total), please visit the HSDB record page.
Parenteral acyclovir therapy can cause signs and symptoms of encephalopathy. ... Acyclovir should be used with caution in patients with underlying neurologic abnormalities and in patients with serious renal, hepatic, or electrolyte abnormalities or substantial hypoxia. The drug also should be used with caution in patients who have manifested prior neurologic reactions to cytotoxic drugs or those receiving intrathecal methotrexate or interferon.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 767
Acyclovir should be used with caution in patients receiving other nephrotoxic drugs concurrently since the risk of acyclovir-induced renal impairment and/or reversible CNS symptoms is increased in these patients. Adequate hydration should be maintained in patients receiving IV acyclovir; however, in patients with encephalitis, the recommended hydration should be balanced by the risk of cerebral edema. Because the risk of acyclovir-induced renal impairment is increased during rapid IV administration of the drug, acyclovir should be given only by slow IV infusion (over 1 hour).
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 767
There are no adequate and controlled studies to date using acyclovir in pregnant women, and the drug should be used during pregnancy only when the potential benefits justify the possible risks to the fetus.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 767
Maternal Medication usually Compatible with Breast-Feeding: Acyclovir: Reported Sign or Symptom in Infant or Effect on Lactation: None. /from Table 6/
Report of the American Academy of Pediatrics Committee on Drugs in Pediatrics 93 (1): 140 (1994)
For more Drug Warnings (Complete) data for ACYCLOVIR (20 total), please visit the HSDB record page.
An acyclovir topical cream is indicated to treat recurrent herpes labialis in immunocompetent patients 12 years and older. Acyclovir oral tablets, capsules, and suspensions are indicated to treat herpes zoster, genital herpes, and chickenpox. An acyclovir topical ointment is indicated to treat initial genital herpes and limited non-life-threatening mucocutaneous herpes simplex in immunocompromised patients. An acyclovir cream with hydrocortisone is indicated to treat recurrent herpes labialis, and shortening lesion healing time in patients 6 years and older. An acyclovir buccal tablet is indicated for the treatment of recurrent herpes labialis. An acyclovir ophthalmic ointment is indicated to treat acute herpetic keratitis.
Treatment of herpes simplex labialis
Prevention of recurrences of herpes simplex labialis, Treatment of recurrent herpes simplex labialis
Acyclovir is an agent used to treat infections caused by the herpes simplex virus (HSV). It is FDA-approved to treat genital herpes and HSV encephalitis. Non-FDA-approved indications are mucocutaneous HSV, herpes zoster (shingles), and varicella zoster (chickenpox). Acyclovir is the first-line treatment for HSV encephalitis. Currently, no other medications are indicated for treating this condition.
Antiviral Agents
Agents used in the prophylaxis or therapy of VIRUS DISEASES. Some of the ways they may act include preventing viral replication by inhibiting viral DNA polymerase; binding to specific cell-surface receptors and inhibiting viral penetration or uncoating; inhibiting viral protein synthesis; or blocking late stages of virus assembly. (See all compounds classified as Antiviral Agents.)
J05AB01
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
S - Sensory organs
S01 - Ophthalmologicals
S01A - Antiinfectives
S01AD - Antivirals
S01AD03 - Aciclovir
D - Dermatologicals
D06 - Antibiotics and chemotherapeutics for dermatological use
D06B - Chemotherapeutics for topical use
D06BB - Antivirals
D06BB03 - Aciclovir
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AB - Nucleosides and nucleotides excl. reverse transcriptase inhibitors
J05AB01 - Aciclovir
S01AD03; J05AB01
Absorption
The oral bioavailability of acyclovir is 10-20% but decreases with increasing doses. Acyclovir ointment is <0.02-9.4% absorbed. Acyclovir buccal tablets and ophthalmic ointment are minimally absorbed. The bioavailability of acyclovir is not affected by food. Acyclovir has a mean Tmax of 1.10.4 hours, mean Cmax of 593.7-656.5ng/mL, and mean AUC of 2956.6-3102.5h/*ng/mL.
Route of Elimination
The majority of acyclovir is excreted in the urine as unchanged drug. 90-92% of the drug can be excreted unchanged through glomerular filtration and tubular secretion. <2% of the drug is recovered in feces and <0.1% is expired as CO2.
Volume of Distribution
The volume of distribution of acyclovir is 0.6L/kg.
Clearance
The renal clearance of acyclovir is 248mL/min/1.73m2. The total clearance in neonates if 105-122mL/min/1.73m2.
Absorption of acyclovir from the GI tract is variable and incomplete. 15-30% of an oral dose of the drug is absorbed. Some data suggest that GI absorption of acyclovir may be saturable; in a crossover study in which acyclovir was administered orally to healthy adults as 200 mg capsules, 400 mg tablets, or 800 mg tablets 6 times daily, the extent of absorption decreased with increasing dose, resulting in bioavailabilities of 20, 15, or 10%, respectively. ... This decrease in bioavailability appears to be a function of increasing dose, not differences in dosage forms. In addition, steady-state peak and trough plasma acyclovir concentrations were not dose proportional over the oral dosing range of 200-800 mg 6 times daily, averaging 0.83 and 0.46, 1.21 and 0.63, or 1.61 and 0.83 ug/ml for the 200, 400, or 800 mg dosing regimens, respectively. Peak plasma concentrations usually occur within 1.5-2.5 hours after oral administration.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 769
In a multiple dose study in neonates up to 3 months of age, IV infusion over 1 hour of 5, 10, or 15 mg/kg of acyclovir every 8 hours resulted in mean steady state peak serum concentrations of 6.8, 13.9, and 19.6 ug/ml, respectively, and mean steady state trough serum concentration of 1.2, 2.3, and 3.1 ug/ml, respectively. In another multiple dose study in pediatric patients, IV infusion over 1 hour of 250 or 500 mg/sq m of acyclovir every 8 hours resulted in mean steady state peak serum concentrations of 10.3 and 20.7 ug/ml, respectively.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 769
Acyclovir is widely distributed into body tissues and fluids including the brain, kidney, saliva, lung, liver, muscle, spleen, uterus, vaginal mucosa and secretions, cerebrospinal fluid, and herpetic vesicular fluid. The drug also is distributed into semen, achieving concentrations about 1.4 and 4 times those in plasma during chronic oral therapy at dosages of 400 mg and 1 g daily, respectively. The apparent volume of distribution of acyclovir is reported to be 32.4-61.8 liter/1.73 sq m in adults and 28.8, 31.6, 42, or 51.2-53.6 liter/1.73 sq m in neonates up to 3 months of age, children 1-2 years; 2-7 years; or 7-12 years of age, respectively.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 769
Acyclovir crosses the placenta. Limited data indicate that the drug is distributed into milk, generally in concentrations greater than concurrent maternal plasma concentrations, possibly via an active transport mechanism.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 769
For more Absorption, Distribution and Excretion (Complete) data for ACYCLOVIR (13 total), please visit the HSDB record page.
Acyclovir is <15% oxidized to 9-carboxymethoxymethylguanine by alcohol dehydrogenase and aldehyde dehydrogenase and 1% 8-hydroxylated to 8-hydroxy-acyclovir by aldehyde oxidase. Acyclovir is becomes acyclovir monophosphate due to the action of viral thymidine kinase. Acyclovir monophosphate is converted to the diphosphate form by guanylate kinase. Acyclovir diphosphate is converted to acyclovir triphosphate by nucleoside diphosphate kinase, pyruvate kinase, creatine kinase, phosphoglycerate kinase, succinyl-CoA synthetase, phosphoenolpyruvate carboxykinase and adenylosuccinate synthetase.
Acyclovir is metabolized partially to 9-carboxymethoxymethylguanine and minimally to 8-hydroxy-9-(2-hydroxyethoxymethyl)guanine. In vitro, acyclovir also is metabolized to acyclovir monophosphate, diphosphate, and triphosphate in cells infected with herpes viruses, principally by intracellular phosphorylation of the drug by virus coded thymidine kinase and several cellular enzymes.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 769
The clearance of acyclovir varies from 2.5-3 hours depending on the creatinine clearance of the patient. The plasma half life of acyclovir during hemodialysis is approximately 5 hours. The mean half life in patients from 7 months to 7 years old is 2.6 hours.
Plasma concentrations of acyclovir appear to decline in a biphasic manner. In adults with normal renal function, the half-life of acyclovir in the initial phase averages 0.34 hours and the half-life in the terminal phase averages 2.1-3.5 hours. In adults with renal impairment, both half-life in the initial phase and half-life in the terminal phase may be prolonged, depending on the degree of renal impairment. In a study in adults with anuria, the half-life in the initial phase of acyclovir averaged 0.71 hours. In several studies, the half-life in the terminal phase of acyclovir averaged 3,3.5, or 19.5 hours in adults with creatinine clearances of 50-80 or 15-50 ml/minute per 1.73 sq m or with anuria, respectively. In patients undergoing hemodialysis, the half-life in the terminal phase of acyclovir during hemodialysis averaged 5.4-5.7 hours.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 769
In neonates, the half-life of acyclovir depends principally on the maturity of renal mechanisms for excretion as determined by gestational age, chronologic age, and weight. In children older than 1 year of age, the half-life of the drug appears to be similar to that of adults. The half-life in the terminal phase averages 3.8-4.1, 1.9, 2.2-2.9, or 3.6 hours in neonates up to 3 months of age, children 1-2 years, 2-12 years, or 12-17 years of age, respectively.
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013, p. 769
Acyclovir is becomes acyclovir monophosphate due to the action of viral thymidine kinase. Acyclovir monophosphate is converted to the diphosphate form by guanylate kinase. Acyclovir diphosphate is converted to acyclovir triphosphate by nucleoside diphosphate kinase, pyruvate kinase, creatine kinase, phosphoglycerate kinase, succinyl-CoA synthetase, phosphoenolpyruvate carboxykinase and adenylosuccinate synthetase. Acyclovir triphosphate has higher affinity for viral DNA polymerase than cellular DNA polymerase and incorporates into the DNA where the missing 2' and 3' carbons causes DNA chain termination. In other cases acyclovir triphosphate competes so strongly for viral DNA polymerase that other bases cannot associate with the enzyme, inactivating it.
Acyclovir is a synthetic purine nucleoside analogue with in vitro and in vivo inhibitory activity against herpes simplex virus types 1 (HSV-1), 2 (HSV-2), and varicella-zoster virus (VZV). The inhibitory activity of acyclovir is highly selective due to is affinity for the enzyme thymidine kinase (TK) encoded by HSV and VZV. This viral enzyme converts acyclovir into acyclovir monophosphate, a nucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase adn into triphosphate by a number of cellualr enzymes. In vitro, acyclovir triphosphate stops replication of herpes viral DNA.
Physicians Desk Reference 65th ed. PDR Network, LLC, Montvale, NJ. 2011, p. 1650
Acyclovir inhibits viral DNA synthesis ... . Its selectivity of action depends on interaction with two distinct viral proteins. Cellular uptake and initial phosphorylation are facilitated by HSV thymidine kinase. The affinity of acyclovir for HSV thymidine kinase is about 200-fold greater than for the mammalian enzyme. Cellular enzymes convert the monophosphate to acyclovir triphosphate, which is present in 40- to 100-fold higher concentrations in HSV-infected than in uninfected cells, and competes for endogenous deoxyguanosine triphosphate (dGTP). The immunosuppressive agent mycophenolate mofetil potentiates the antiherpes activity of acyclovir and related agents by depleting intracellular dGTP pools. Acyclovir triphosphate competitively inhibits viral DNA polymerases and, to a much smaller extent, cellular DNA polymerases. Acyclovir triphosphate also is incorporated into viral DNA, where it acts as a chain terminator because of the lack of 3'-hydroxyl group. By a mechanism termed suicide inactivation, the terminated DNA template containing acyclovir binds the enzyme and leads to irreversible inactivation of the DNA polymerase.
Brunton, L. Chabner, B, Knollman, B. Goodman and Gillman's The Pharmaceutical Basis of Therapeutics, Twelth Edition, McGraw Hill Medical, New York, NY. 2011, p. 1594
The concentration of the endogenous neurotoxin quinolinic acid (QA) is increased in the central nervous system of mice with herpes simplex encephalitis. /The authors/ have previously shown that the antiherpetic agent acyclovir (AC) has the ability to reduce QA-induced neuronal damage in rat brain, by attenuating lipid peroxidation. The mechanism by which QA induces lipid peroxidation includes the enhancement of the iron (Fe)-mediated Fenton reaction and the generation of free radicals, such as the superoxide anion (O(2)(-)). Thus, the present study determined whether AC has the ability to reduce Fe(2+)-induced lipid peroxidation, O(2)(-) generation and QA-induced superoxide anion generation, and to bind free Fe. O(2)(-) and Fe(2+) are also cofactors of the enzymes, indoleamine-2,3-dioxygenase (IDO) and 3-hydroxyanthranilate-3,4-dioxygenase (3-HAO) respectively. These enzymes catalyse steps in the biosynthesis of QA; thus, the effect of AC on their activity was also investigated. AC significantly attenuates Fe(2+)-induced lipid peroxidation and O(2)(-) generation. AC reduces O(2)(-) generation in the presence of QA and strongly binds Fe(2+) and Fe(3+). It also reduces the activity of both IDO and 3-HAO, which could be attributed to the superoxide anion scavenging and iron binding properties, respectively, of this drug.
PMID:17174341 Muller AC et al; Life Sci 80 (10): 918-25 (2007)

