




1. 241479-74-3
2. Isavuconazole Impurity 6
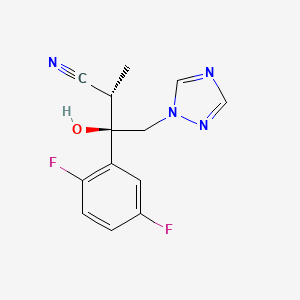
3. (2s,3r)-3-(2,5-difluorophenyl)-3-hydroxy-2-methyl-4-(1,2,4-triazol-1-yl)butanenitrile
4. (alphas,betar)-beta-(2,5-difluorophenyl)-beta-hydroxy-alpha-methyl-1h-1,2,4-triazole-1-butanenitrile
5. Schembl1656831
6. Dtxsid401129526
7. 1286729-93-8
8. Cs-z0013
9. Mfcd28411477
10. Zinc81956485
11. Akos027250773
12. Ds-9076
13. Ac-31068
14. Cs-0012464
15. A854829
16. (2s,3r)-3-(2,5-difluoro-phenyl)-3-hydroxy-2-methyl-4-[1,2,4]triazol-1-yl-butyronitrile
17. 3-(2,5-difluoro-phenyl)-3-hydroxy-2-methyl-4-[1,2,4]triazol-1-yl-butyronitrile
18. Rel-(2r,3s)-3-(2,5-difluorophenyl)-3-hydroxy-2-methyl-4-(1h-1,2,4-triazol-1-yl)butanenitrile
| Molecular Weight | 278.26 g/mol |
|---|---|
| Molecular Formula | C13H12F2N4O |
| XLogP3 | 1.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 4 |
| Exact Mass | 278.09791734 g/mol |
| Monoisotopic Mass | 278.09791734 g/mol |
| Topological Polar Surface Area | 74.7 Ų |
| Heavy Atom Count | 20 |
| Formal Charge | 0 |
| Complexity | 388 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


