04 Dec 2025
// PRESS RELEASE
29 Jun 2024
// PRESS RELEASE
18 Jan 2024
// PRESS RELEASE
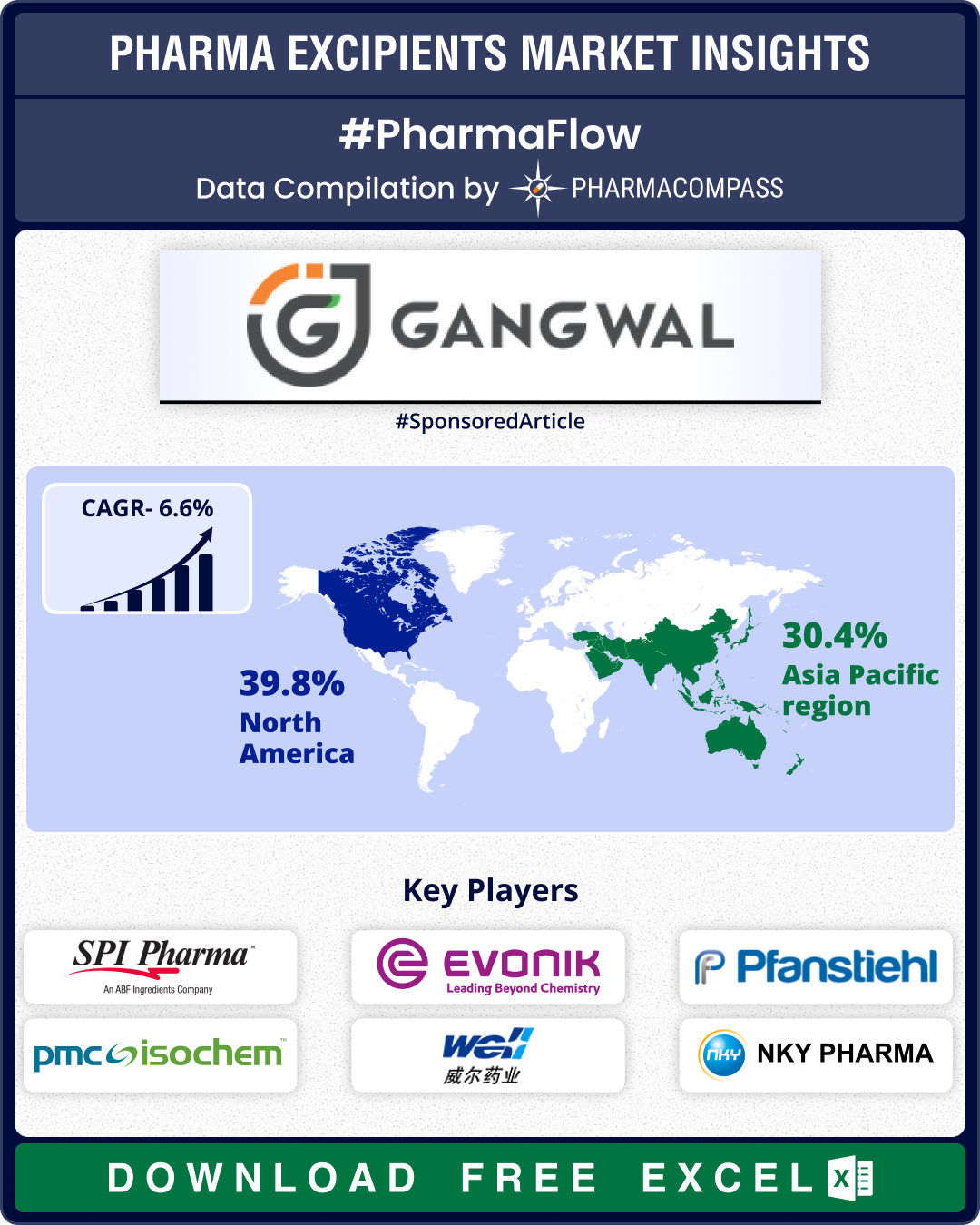
 KEY EXCIPIENTS
KEY EXCIPIENTS
Boai NKY Pharmaceuticals Ltd. is one of the largest global PVP suppliers.
About
Industry Trade Show
Not Confirmed
26-28 February, 2026
euroPLX 90 LisboneuroPLX 90 Lisbon
Industry Trade Show
Not Confirmed
02-03 March, 2026
BIO CEO & InvestorBIO CEO & Investor
Industry Trade Show
Not Confirmed
02-03 March, 2026
CONTACT DETAILS




Facebook: https://www.facebook.com/profile.php?id=100093970467910
Youtube: https://www.youtube.com/@BoaiNKY
Instagram: https://www.instagram.com/boainky/
Events
Webinars & Exhibitions
Industry Trade Show
Not Confirmed
26-28 February, 2026
euroPLX 90 LisboneuroPLX 90 Lisbon
Industry Trade Show
Not Confirmed
02-03 March, 2026
BIO CEO & InvestorBIO CEO & Investor
Industry Trade Show
Not Confirmed
02-03 March, 2026
https://www.pharmacompass.com/speak-pharma/we-owe-our-success-to-our-focus-on-r-d-quality-and-better-service
CORPORATE CONTENT #SupplierSpotlight
https://www.pharmacompass.com/radio-compass-blog/excipient-market-overview-roquette-announces-restructuring-post-iff-pharma-buyout-who-fda-advance-regulatory-frameworks
https://www.pharmacompass.com/radio-compass-blog/excipient-market-overview-roquette-seqens-evonik-make-strategic-moves-new-guidelines-deal-with-contamination

04 Dec 2025
// PRESS RELEASE
https://www.boai-nky.com/news/nky-group-receives-iso14067-certifications-for-8-key-products-from-bureau-veritas.html

29 Jun 2024
// PRESS RELEASE

18 Jan 2024
// PRESS RELEASE

15 Apr 2022
// PRESS RELEASE

08 Apr 2019
// PRESS RELEASE

03 Apr 2019
// PRESS RELEASE
Excipients
Excipients by Ingredients
Excipients By applications
ABOUT THIS PAGE
Boai NKY Pharmaceuticals Ltd is a supplier offers 1 products (APIs, Excipients or Intermediates).
Find a price of Povidone Iodine bulk with DMF, CEP offered by Boai NKY Pharmaceuticals Ltd


 Boai NKY Pharmaceuticals Ltd
Boai NKY Pharmaceuticals Ltd