NDC Code(s) : 81952-113-01, 81952-113-06, 81952-113-03, 81952-113-08, 81952-116-01, 81952-116-06, 81952-112-01, 81952-112-06, 81952-112-04, 81952-112-09, 81952-112-05, 81952-112-10, 81952-111-01, 81952-111-06, 81952-115-02, 81952-115-07, 81952-114-04, 81952-114-09
Packager : Hepalink USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Heparin SodiumHeparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Heparin SodiumHeparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Heparin SodiumHeparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Heparin SodiumHeparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Heparin SodiumHeparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Heparin SodiumHeparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Hepalink USA Inc.(079558168) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Shenzhen Techdow Pharmaceutical Co., Ltd. | 527809171 | manufacture(81952-111, 81952-115, 81952-112, 81952-113, 81952-116, 81952-114), analysis(81952-111, 81952-115, 81952-112, 81952-113, 81952-116, 81952-114) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Shenzhen Hepalink Pharmaceutical Group Co., Ltd. | 547034665 | api manufacture(81952-115, 81952-112, 81952-111, 81952-113, 81952-116, 81952-114) | |
PRINCIPAL DISPLAY PANEL
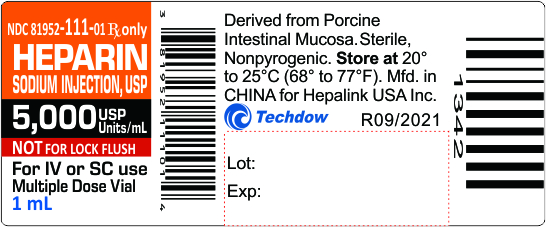
NDC 81952-111-01 Rx only
HEPARIN
SODIUM INJECTION, USP
5,000 USP Units/mL
NOT FOR LOCK FLUSH
For IV or SC use
Multiple Dose Vial
1 mL
PRINCIPAL DISPLAY PANEL
NDC 81952-111-06 Rx only
HEPARIN
SODIUM INJECTION, USP
5,000 USP Units/mL
NOT FOR LOCK FLUSH
For Intravenous or Subcutaneous use
25 Multiple Dose Vials
1 mL
Derived from Porcine Intestinal Mucosa

PRINCIPAL DISPLAY PANEL
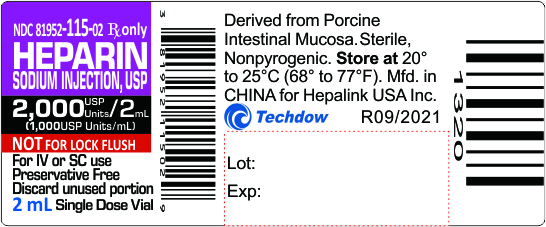
NDC 81952-115-02 Rx only
HEPARIN
SODIUM INJECTION, USP
2,000 USP Units/2mL
(1,000USP Units/mL)
NOT FOR LOCK FLUSH
For IV or SC use
Preservative Free
Discard unused portion
2mL Single Dose Vial
PRINCIPAL DISPLAY PANEL
NDC 81952-115-07 Rx only
HEPARIN
SODIUM INJECTION, USP
2,000 USP Units/2mL
(1,000USP Units/mL)
NOT FOR LOCK FLUSH
For Intravenous or Subcutaneous use
Preservative Free
25 Single Dose Vials
2 mL
Derived from Porcine Intestinal Mucosa

PRINCIPAL DISPLAY PANEL
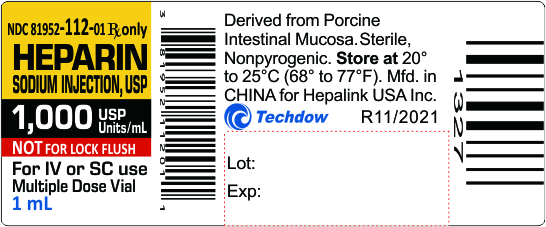
NDC 81952-112-01 Rx only
HEPARIN
SODIUM INJECTION, USP
1,000 USP Units/mL
NOT FOR LOCK FLUSH
For IV or SC use
Multiple Dose Vial
1mL
PRINCIPAL DISPLAY PANEL
NDC 81952-112-06 Rx only
HEPARIN
SODIUM INJECTION, USP
1,000 USP Units/mL
NOT FOR LOCK FLUSH
For Intravenous or Subcutaneous use
25 Multiple Dose Vials
1 mL
Derived from Porcine Intestinal Mucosa

PRINCIPAL DISPLAY PANEL
NDC 81952-112-04 Rx only
HEPARIN
SODIUM INJECTION, USP
10,000 USP Units/10mL
(1,000USP Units/mL)
NOT FOR LOCK FLUSH
For IV or SC use
Multiple Dose Vial
10mL

PRINCIPAL DISPLAY PANEL
NDC 81952-112-09 Rx only
HEPARIN
SODIUM INJECTION, USP
10,000 USP Units/10mL
(1,000USP Units/mL)
NOT FOR LOCK FLUSH
For Intravenous or Subcutaneous use
25 Multiple Dose Vials
10 mL
Derived from Porcine Intestinal Mucosa

PRINCIPAL DISPLAY PANEL
NDC 81952-113-01 Rx only
HEPARIN
SODIUM INJECTION, USP
10,000 USP Units/mL
NOT FOR LOCK FLUSH
For IV or SC use
Multiple Dose Vial
1mL
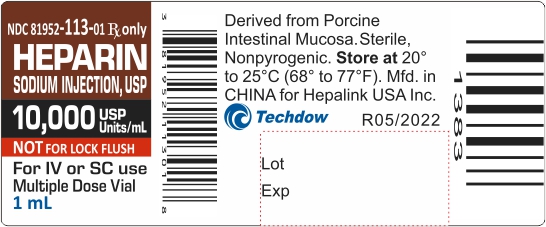
PRINCIPAL DISPLAY PANEL
NDC 81952-113-06 Rx only
HEPARIN
SODIUM INJECTION, USP
10,000 USP Units/mL
NOT FOR LOCK FLUSH
For Intravenous or Subcutaneous use
25 Multiple Dose Vials
1 mL
Derived from Porcine Intestinal Mucosa

PRINCIPAL DISPLAY PANEL
NDC 81952-116-01 Rx only
HEPARIN
SODIUM INJECTION, USP
20,000 USP Units/mL
NOT FOR LOCK FLUSH
For IV or SC use
Multiple Dose Vial
1mL

PRINCIPAL DISPLAY PANEL
NDC 81952-116-06 Rx only
HEPARIN
SODIUM INJECTION, USP
20,000 USP Units/mL
NOT FOR LOCK FLUSH
For Intravenous or Subcutaneous use
25 Multiple Dose Vials
1 mL
Derived from Porcine Intestinal Mucosa

PRINCIPAL DISPLAY PANEL
NDC 81952-112-05 Rx only
HEPARIN
SODIUM INJECTION, USP
30,000 USP Units/30mL
(1,000 USP Units/mL)
NOT FOR LOCK FLUSH
For IV or SC use
Multiple Dose Vial
30mL

PRINCIPAL DISPLAY PANEL
NDC 81952-112-10 Rx only
HEPARIN
SODIUM INJECTION, USP
30,000 USP Units/30mL
(1,000USP Units/mL)
NOT FOR LOCK FLUSH
For Intravenous or Subcutaneous use
25 Multiple Dose Vials
30 mL
Derived from Porcine Intestinal Mucosa

PRINCIPAL DISPLAY PANEL
NDC 81952-113-03 Rx only
HEPARIN
SODIUM INJECTION, USP
50,000 USP Units/5mL
(10,000USP Units/mL)
NOT FOR LOCK FLUSH
For IV or SC use
Multiple Dose Vial
5mL

PRINCIPAL DISPLAY PANEL
NDC 81952-113-08 Rx only
HEPARIN
SODIUM INJECTION, USP
50,000 USP Units/5mL
(10,000 USP Units/mL)
NOT FOR LOCK FLUSH
For Intravenous or Subcutaneous use
25 Multiple Dose Vials
5 mL
Derived from Porcine Intestinal Mucosa

PRINCIPAL DISPLAY PANEL
NDC 81952-114-04 Rx only
HEPARIN
SODIUM INJECTION, USP
50,000 USP Units/10mL
(5,000 USP Units/mL)
NOT FOR LOCK FLUSH
For IV or SC use
Multiple Dose Vial
10mL

PRINCIPAL DISPLAY PANEL
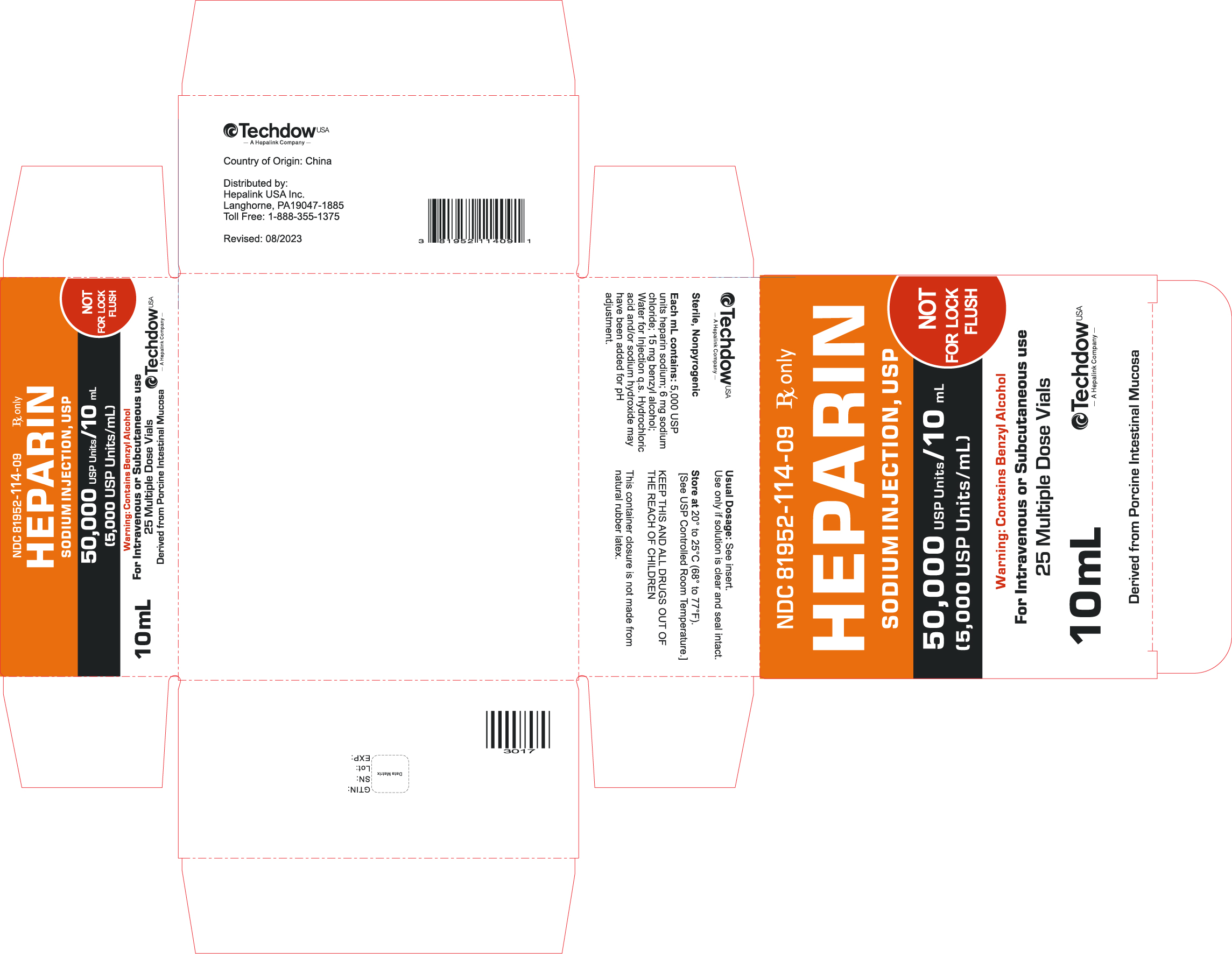
NDC 81952-114-09 Rx only
HEPARIN
SODIUM INJECTION, USP
50,000 USP Units/10mL
(5,000 USP Units/mL)
NOT FOR LOCK FLUSH
For Intravenous or Subcutaneous use
25 Multiple Dose Vials
10 mL
Derived from Porcine Intestinal Mucosa