NDC Code(s) : 76237-304-30, 76237-304-39, 76237-307-30, 76237-307-39, 76237-309-30, 76237-309-39, 76237-312-30, 76237-312-39, 76237-314-30, 76237-314-39
Packager : Mckesson Contract Packaging
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Lisinopril 2.5mgLisinopril TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Lisinopril 5mgLisinopril TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Lisinopril 10mgLisinopril TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Lisinopril 20mgLisinopril TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Lisinopril 40mgLisinopril TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
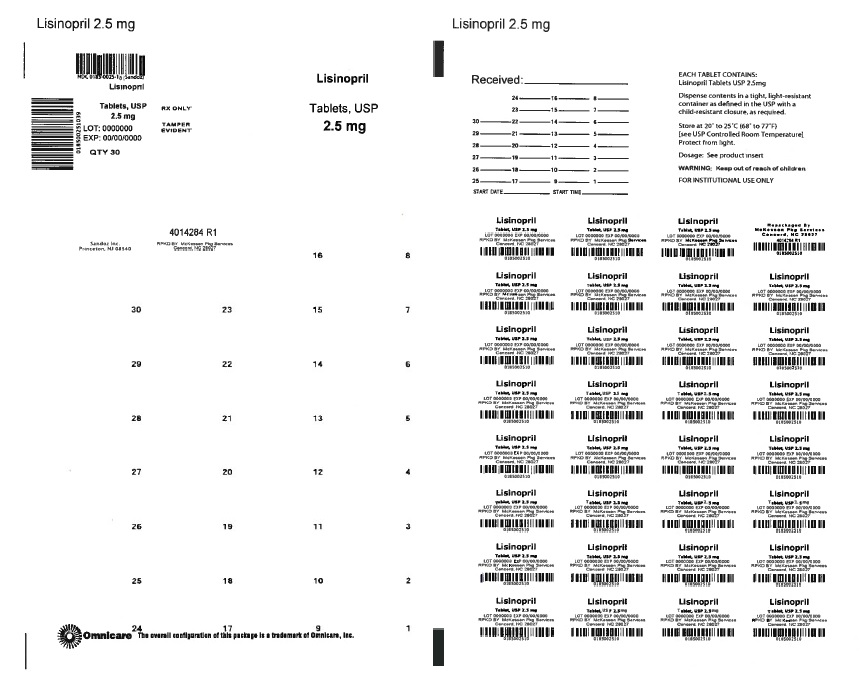
PRINCIPAL DISPLAY PANEL

NDC 76237-304-30
Lisinopril Tablets USP, 2.5 mg
Each tablet contains Lisinopril USP...... 2.5 mg
Warning: Keep out of reach of Children.
Store at 20°-25°C (68°-77°F)[See USP controlled room temperature]
Dispense in a tight, light- resistant container.
PROTECT FROM LIGHT AND MOISTURE.
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL

NDC 76237-307-30
Lisinopril Tablets USP, 5 mg
Each tablet contains Lisinopril USP...... 5 mg
Warning: Keep out of reach of Children.
Store at 20°-25°C (68°-77°F)[See USP controlled room temperature]
Dispense in a tight, light- resistant container.
PROTECT FROM LIGHT AND MOISTURE.
PRINCIPAL DISPLAY PANEL

PRINCIPAL DISPLAY PANEL

NDC 76237-309-30
Lisinopril Tablets USP, 10 mg
Each tablet contains Lisinopril USP...... 10 mg
Warning: Keep out of reach of Children.
Store at 20°-25°C (68°-77°F)[See USP controlled room temperature]
Dispense in a tight, light- resistant container.
PROTECT FROM LIGHT AND MOISTURE.
PRINCIPAL DISPLAY PANEL

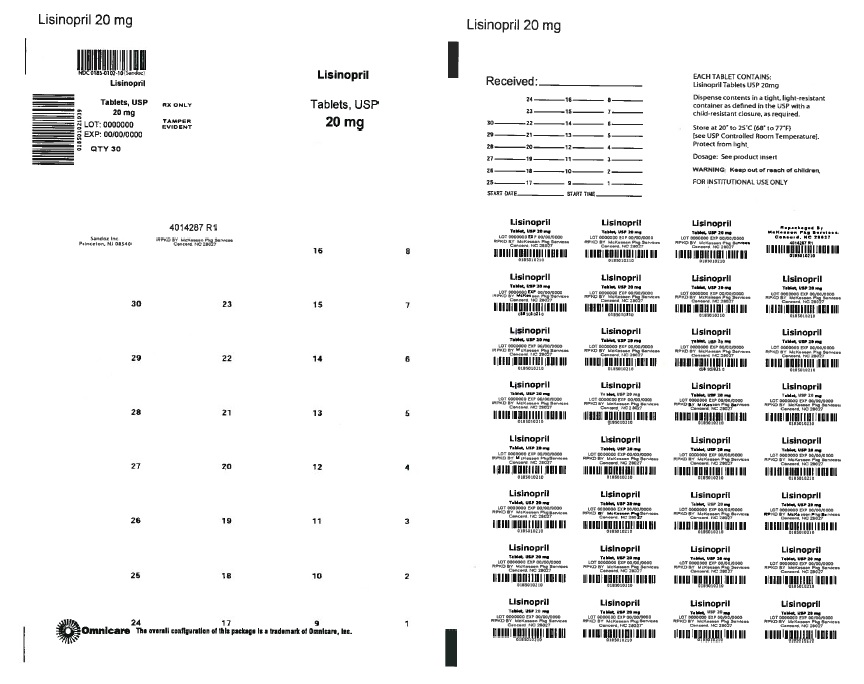
PRINCIPAL DISPLAY PANEL

NDC 76237-312-30
Lisinopril Tablets USP, 20 mg
Each tablet contains Lisinopril USP...... 20 mg
Warning: Keep out of reach of Children.
Store at 20°-25°C (68°-77°F)[See USP controlled room temperature]
Dispense in a tight, light- resistant container.
PROTECT FROM LIGHT AND MOISTURE.
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL

NDC 76237-314-30
Lisinopril Tablets USP, 40 mg
Each tablet contains Lisinopril USP...... 40 mg
Warning: Keep out of reach of Children.
Store at 20°-25°C (68°-77°F)[See USP controlled room temperature]
Dispense in a tight, light- resistant container.
PROTECT FROM LIGHT AND MOISTURE.
PRINCIPAL DISPLAY PANEL










