NDC Code(s) : 76204-200-25, 76204-200-30, 76204-200-60, 76204-200-01
Packager : Ritedose Pharmaceuticals, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Albuterol SulfateAlbuterol Sulfate SOLUTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Ritedose Pharmaceuticals, LLC(968062294) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| The Ritedose Corporation | 837769546 | label(76204-200), manufacture(76204-200), pack(76204-200), analysis(76204-200) | |
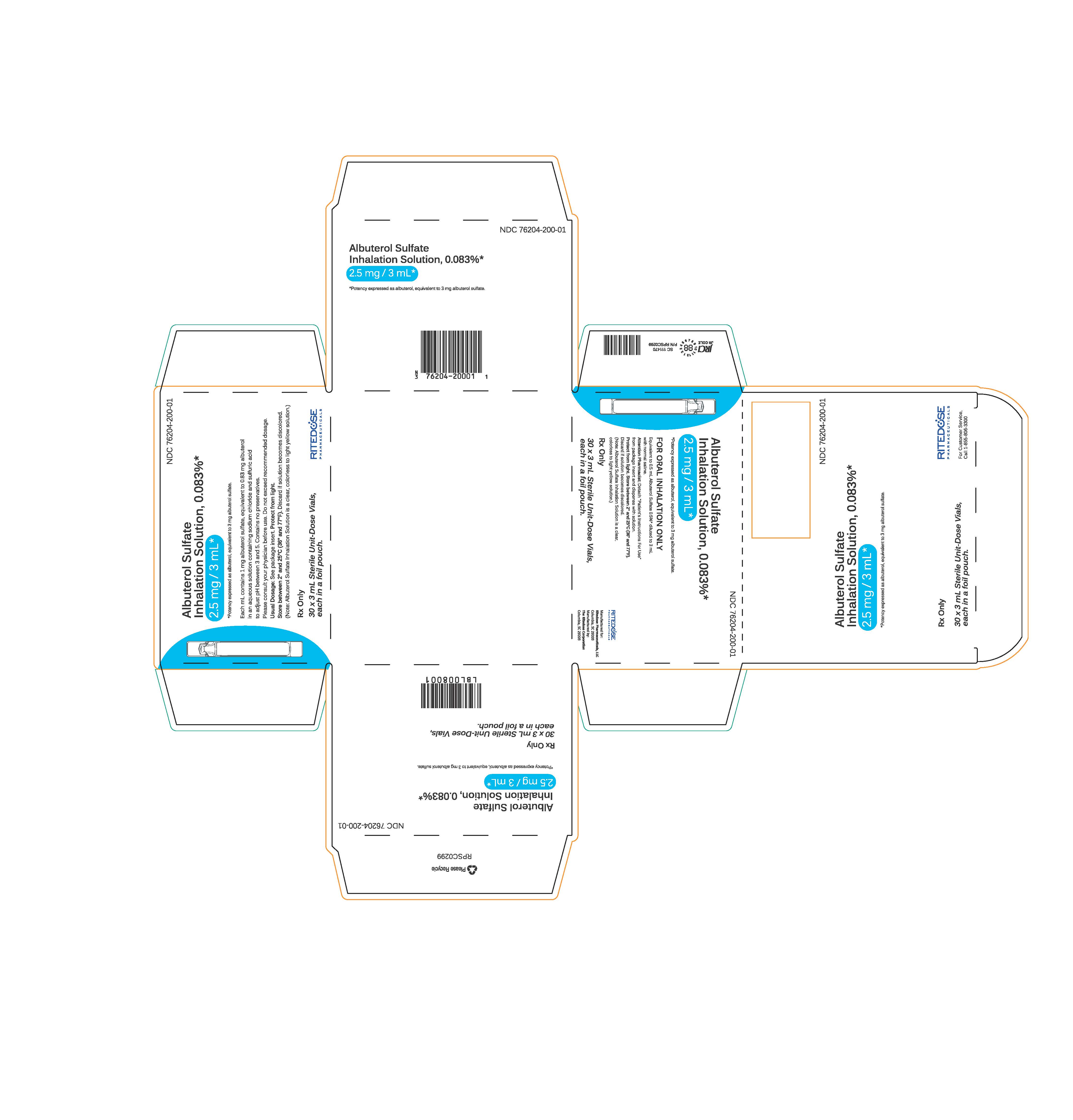
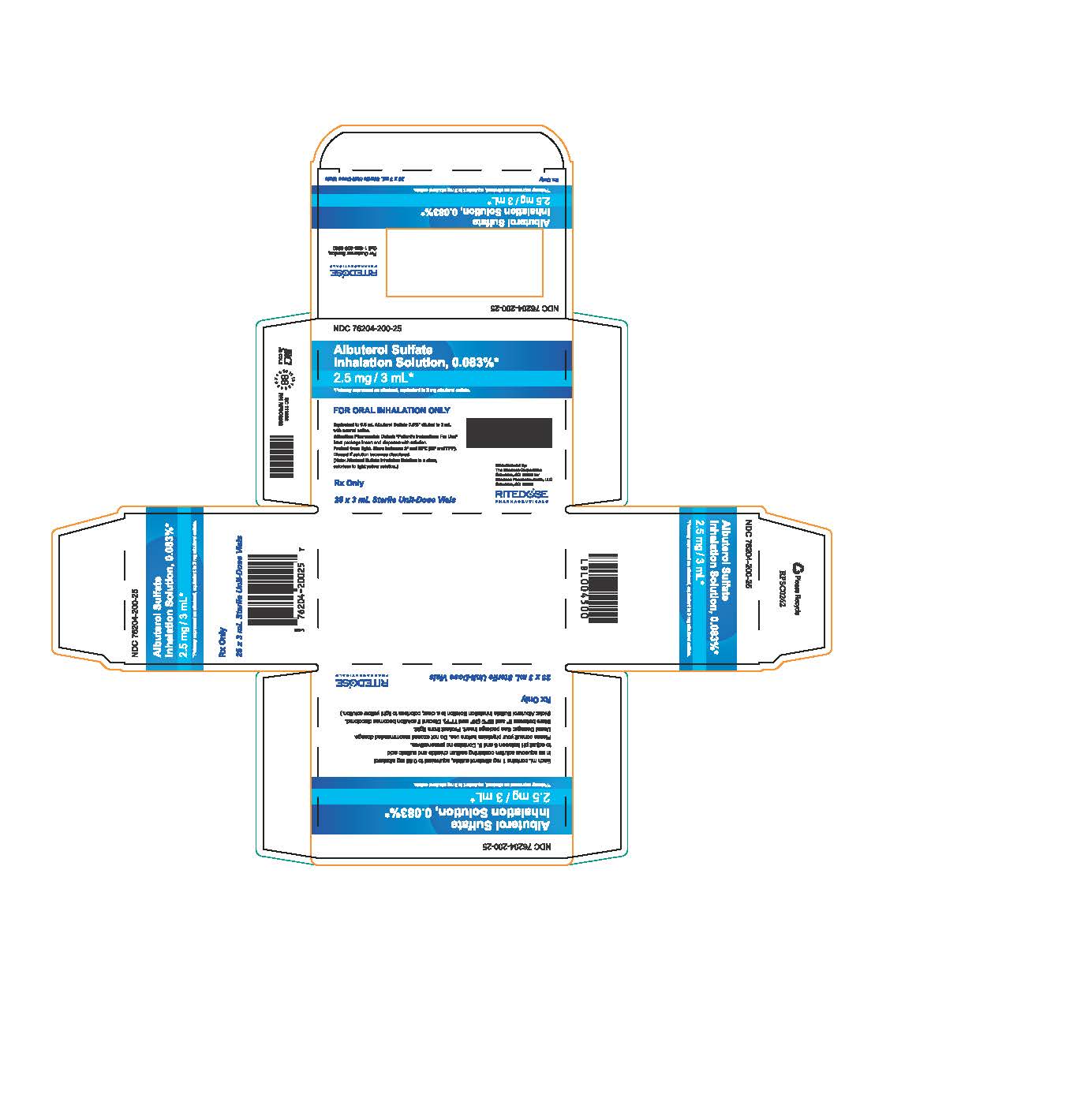
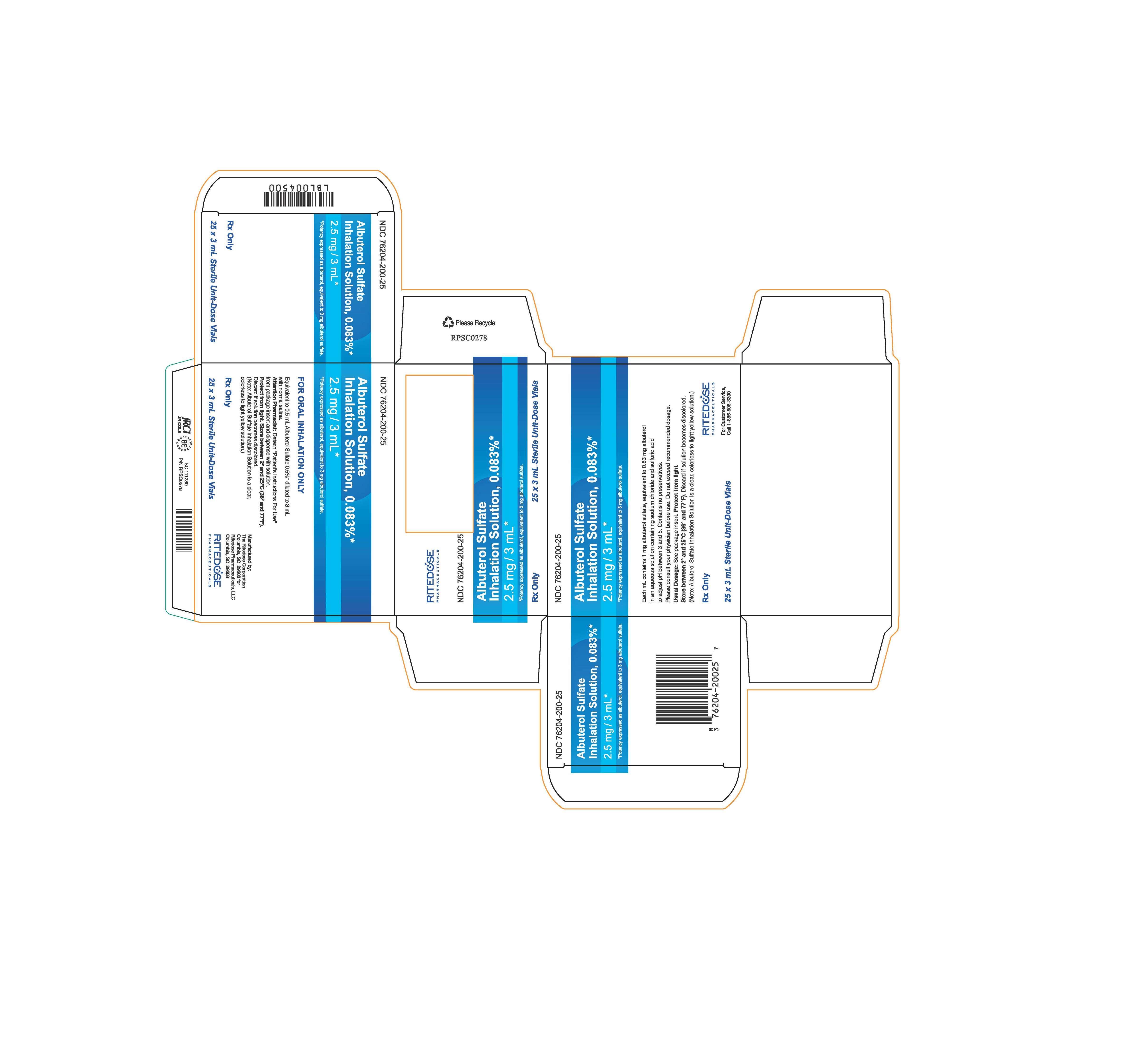
PRINCIPAL DISPLAY PANEL
NDC 76204-200-25
Albuterol Sulfate
Inhalation Solution, 0.083%*
2.5 mg / 3 mL*
*Potency expressed as albuterol, equivalent to 3 mg albuterol sulfate.
Each mL contains 1 mg albuterol sulfate, equivalent to 0.83 mg albuterol
in an aqueous solution containing sodium chloride and sulfuric acid
to adjust pH between 3 and 5. Contains no preservatives.
Please consult your physician before use. Do not exceed recommended dosage.
Usual Dosage: See package insert.
Protect from light.
Store between 2° and 25°C (36° and 77°F). Discard if solution becomes discolored.
(Note: Albuterol Sulfate Inhalation Solution is a clear, colorless to light yellow solution.)
Rx Only
25 x 3 mL Sterile Unit-Dose Vials
RITEDOSE PHARMACEUTICALS
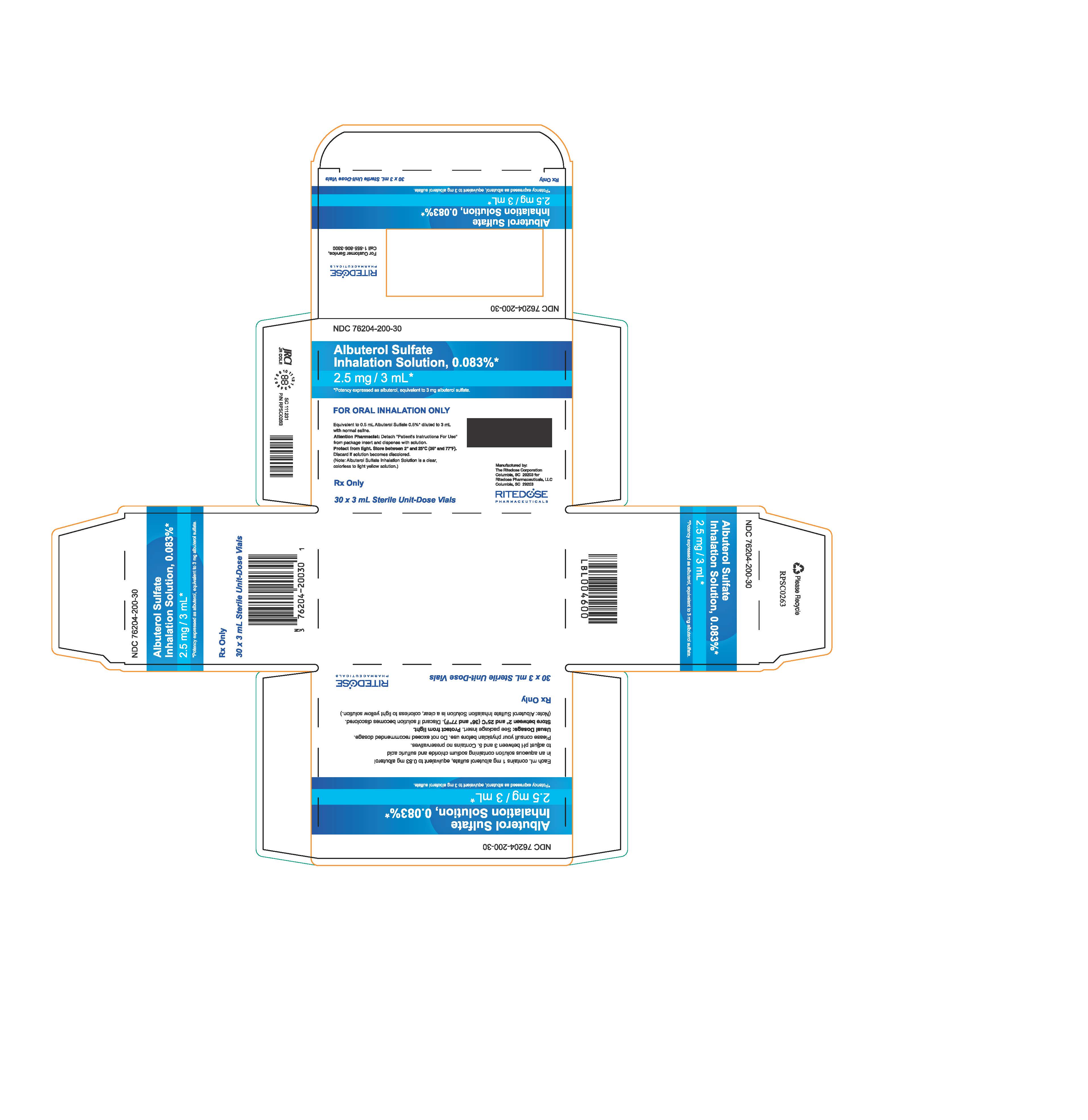
PRINCIPAL DISPLAY PANEL
NDC 76204-200-30
Albuterol Sulfate
Inhalation Solution, 0.083%*
2.5 mg / 3 mL*
*Potency expressed as albuterol, equivalent to 3 mg albuterol sulfate.
Each mL contains 1 mg albuterol sulfate, equivalent to 0.83 mg albuterol
in an aqueous solution containing sodium chloride and sulfuric acid
to adjust pH between 3 and 5. Contains no preservatives.
Please consult your physician before use. Do not exceed recommended dosage.
Usual Dosage: See package insert.
Protect from light.
Store between 2° and 25°C (36° and 77°F). Discard if solution becomes discolored.
(Note: Albuterol Sulfate Inhalation Solution is a clear, colorless to light yellow solution.)
Rx Only
30 x 3 mL Sterile Unit-Dose Vials
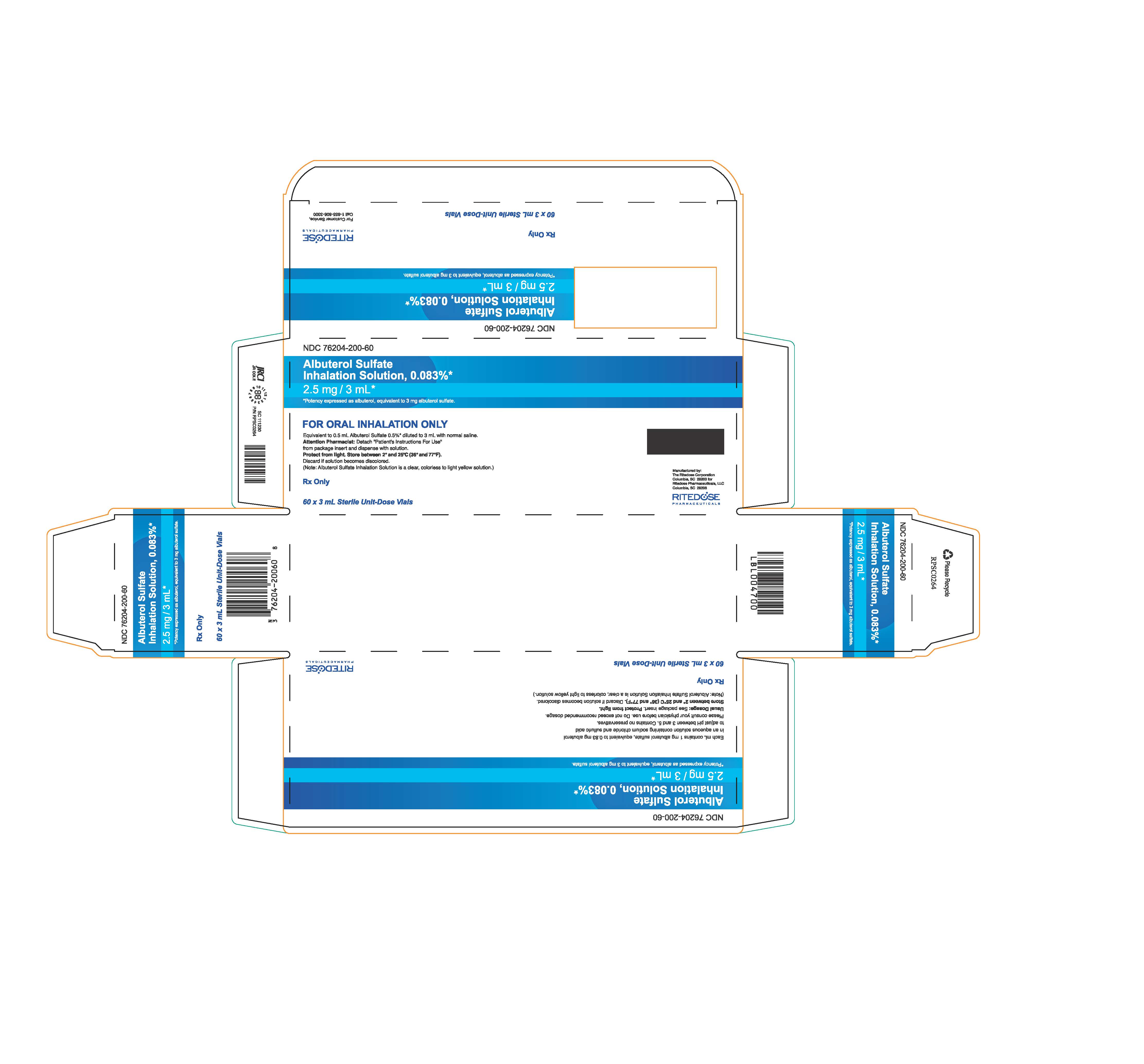
PRINCIPAL DISPLAY PANEL
NDC 76204-200-60
Albuterol Sulfate
Inhalation Solution, 0.083%*
2.5 mg / 3 mL*
*Potency expressed as albuterol, equivalent to 3 mg albuterol sulfate.
Each mL contains 1 mg albuterol sulfate, equivalent to 0.83 mg albuterol
in an aqueous solution containing sodium chloride and sulfuric acid
to adjust pH between 3 and 5. Contains no preservatives.
Please consult your physician before use. Do not exceed recommended dosage.
Usual Dosage: See package insert.
Protect from light.
Store between 2° and 25°C (36° and 77°F). Discard if solution becomes discolored.
(Note: Albuterol Sulfate Inhalation Solution is a clear, colorless to light yellow solution.)
Rx Only
60 x 3 mL Sterile Unit-Dose Vials
PRINCIPAL DISPLAY PANEL
NDC 76204-200-25
Albuterol Sulfate
Inhalation Solution, 0.083%*
2.5 mg / 3 mL*
*Potency expressed as albuterol, equivalent to 3 mg albuterol sulfate.
Each mL contains 1 mg albuterol sulfate, equivalent to 0.83 mg albuterol
in an aqueous solution containing sodium chloride and sulfuric acid
to adjust pH between 3 and 5. Contains no preservatives.
Please consult your physician before use. Do not exceed recommended dosage.
Usual Dosage: See package insert.
Protect from light.
Store between 2° and 25°C (36° and 77°F). Discard if solution becomes discolored.
(Note: Albuterol Sulfate Inhalation Solution is a clear, colorless to light yellow solution.)
Rx Only
25 x 3 mL Sterile Unit-Dose Vials
PRINCIPAL DISPLAY PANEL
NDC 76204-200-01
Albuterol Sulfate
Inhalation Solution, 0.083%*
2.5 mg / 3 mL*
*Potency expressed as albuterol, equivalent to 3 mg albuterol sulfate.
Each mL contains 1 mg albuterol sulfate, equivalent to 0.83 mg albuterol
in an aqueous solution containing sodium chloride and sulfuric acid
to adjust pH between 3 and 5. Contains no preservatives.
Please consult your physician before use. Do not exceed recommended dosage.
Usual Dosage: See package insert.
Protect from light.
Store between 2° and 25°C (36° and 77°F). Discard if solution becomes discolored.
(Note: Albuterol Sulfate Inhalation Solution is a clear, colorless to light yellow solution.)
Rx Only
30 x 3 mL Sterile Unit-Dose Vials