NDC Code(s) : 76112-520-30
Packager : Jubilant HollisterStier General Partnership
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Pentetic Acidpentetic acid INJECTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
PRINCIPAL DISPLAY PANEL
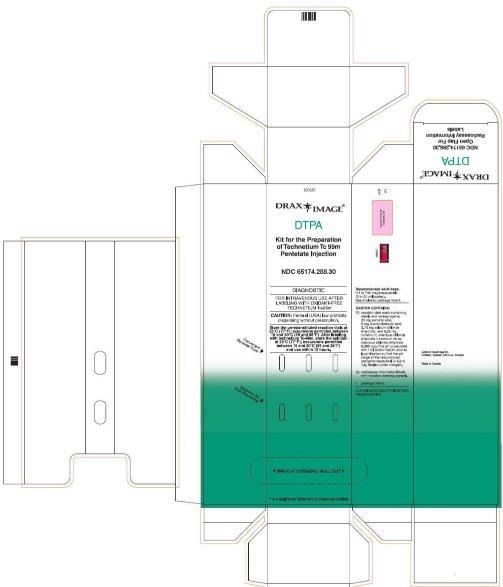 DTPA 30 count
DTPA 30 count









