NDC Code(s) : 74725-100-25, 74725-100-64
Packager : Telix Pharmaceuticals (US) Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ILLUCCIXKit for the preparation of gallium Ga 68 gozetotide injection KIT | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Telix Pharmaceuticals (US) Inc.(116991792) |
PRINCIPAL DISPLAY PANEL
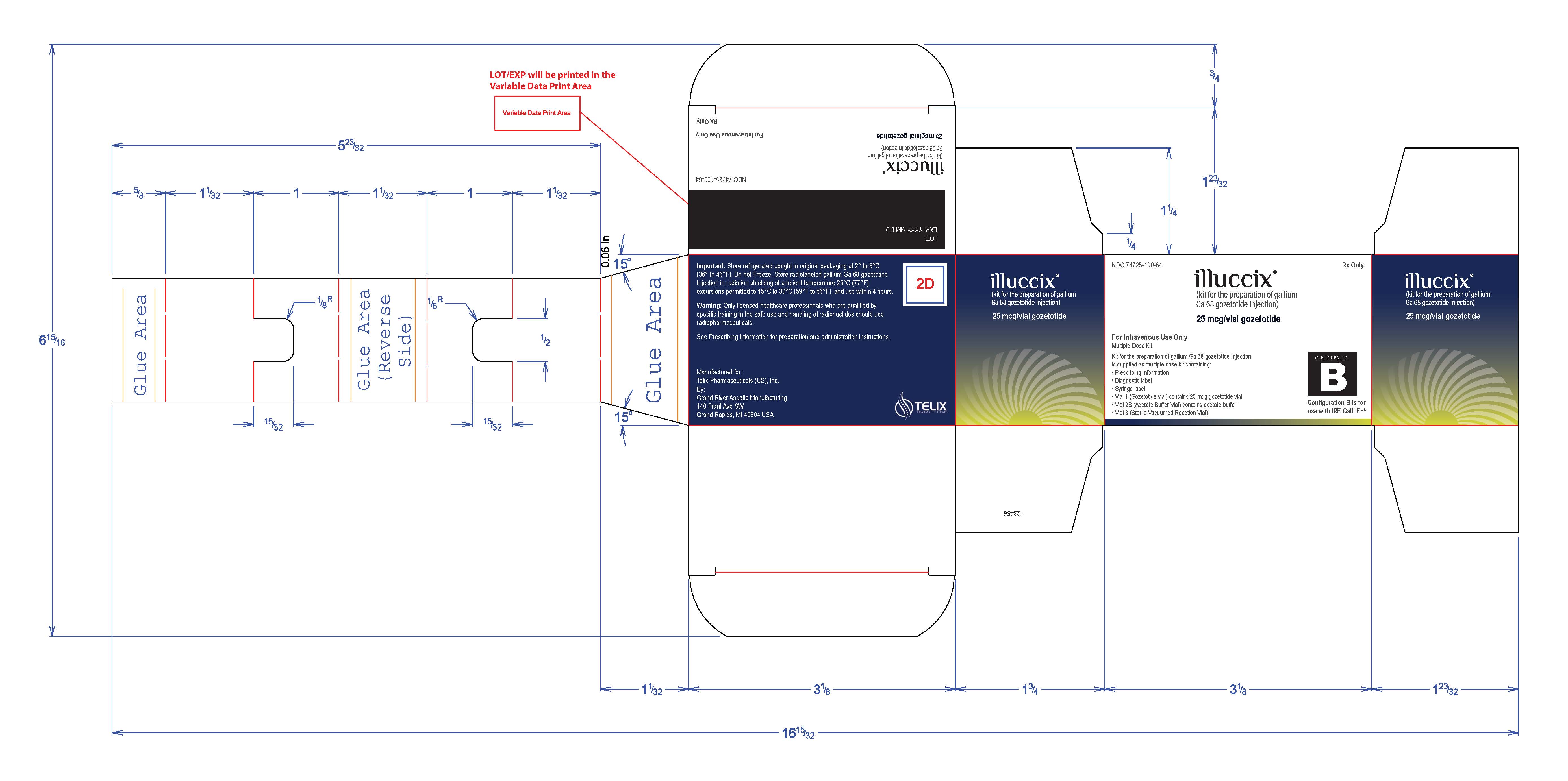
NDC 74725-100-25
Rx-Only
illuccix
®
(kit for the preparation of gallium
Ga 68 gozetotide Injection)
25 mcg/vial gozetotide
For Intravenous Use Only
Multi-Dose Kit
Kit for the preparation of gallium Ga 68 gozetotide Injection
is supplied as multiple dose kit containing:
- Prescribing Information
- Diagnostic label
- Syringe label
- Vial 1 (Gozetotide vial) contains 25 mcg gozetotide vial
- Vial 2A (Acetate Buffer Vial) contains acetate buffer
- Vial 3 (Sterile Vacuumed Reaction Vial)
Configuration A is for use with
Ga 68 produced via cyclotron
or EZAG GalliaPharm
®
Lot no:
Expiry: YYYY-MM-DD
Important: Store refrigerated upright in original packaging at 2 o to 8 oC (36 o to 46 oF). Do not Freeze. Store radiolabeled gallium Ga 68 gozetotide Injection in radiation shielding at ambient temperature 25 oC (77 oF);
excursions permitted to 15 oC to 30 oC (59 oF to 86 oF), and use within 4 hours.
Warning: Only licensed healthcare professionals who are qualified by specific training in the safe use and handling of radionuclides should use radiopharmaceuticals.
See Prescribing Information for preparation and administration instructions.
Manufactured for:
Telix Pharmaceuticals (US), Inc.
By:
Grand River Aspetic Manufacturing
140 Front Ave SW
Grand Rapids, MI 49504 USA
PRINCIPAL DISPLAY PANEL
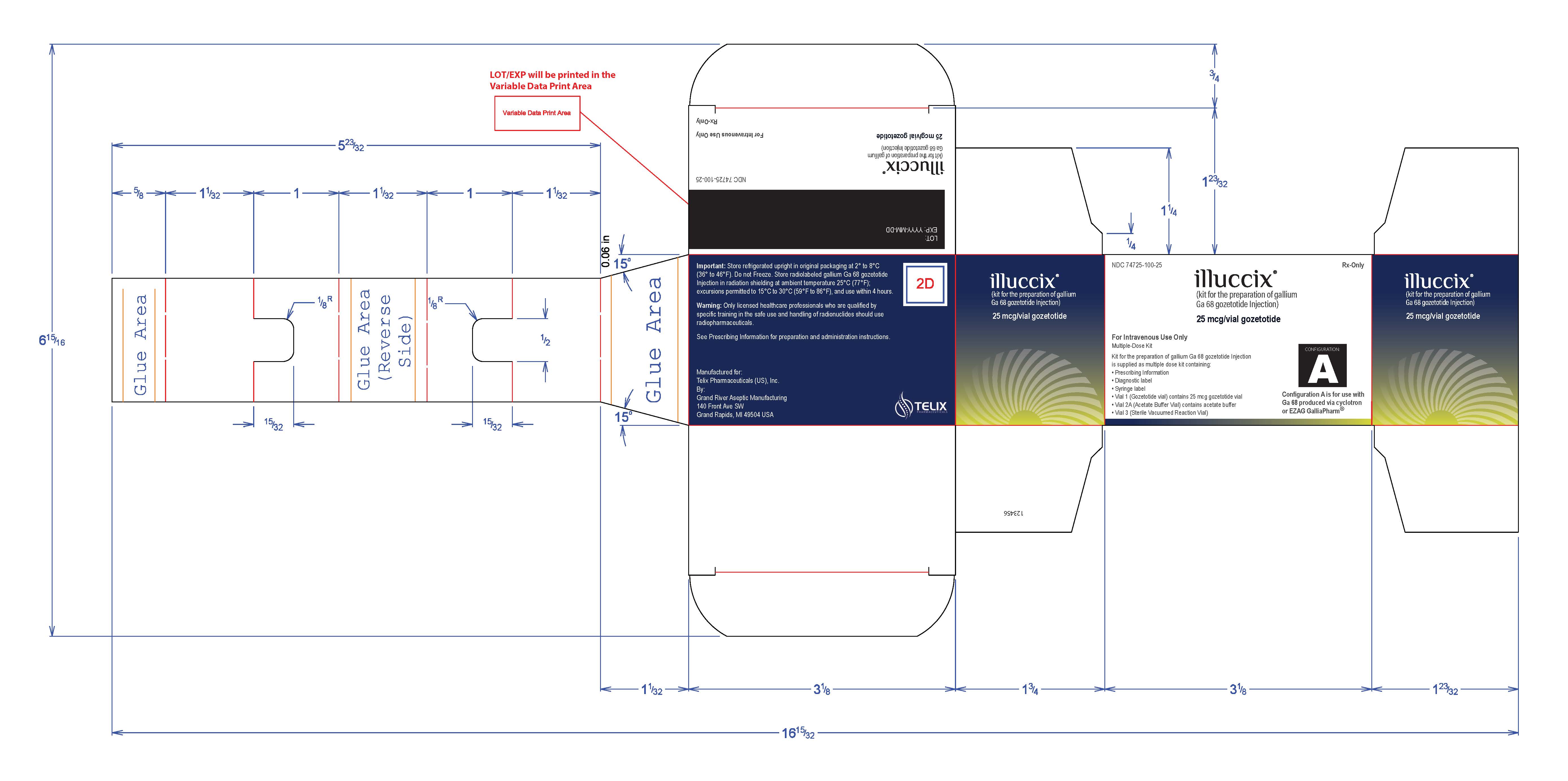
NDC 74725-100-64
Rx Only
illuccix ®
(kit for the preparation of gallium
Ga68 gozetotide Injection)
25 mcg/vial gozetotide
For Intravenous Use Only
Multiple-Dose Kit
Kit for the preparation of gallium Ga 68 gozetotide Inject is supplied as multiple dose kit
containing:
- Prescribing Information
- Diagnostic label
- Syringe label
- Vial 1 (Gozetotide vial) contains 25 mcg gozetotide vial
- Vial 2B (Acetate Buffer Vial) contains acetate buffer
- Vial 3 (Sterile Vacuumed Reaction Vial)
Configuration B is for use with IRE Galli Eo ®
Lot no:
Expiry: YYYY-MM-DD
Important: Store refrigerated upright in original packaging at 2 o to 8 oC (36 o to 46 oF). Do not Freeze. Store radiolabeled gallium Ga 68 gozetotide Injection in radiation shielding at ambient temperature 25oC (77oF);
excursions permitted to 15 oC to 30 oC (59 oF to 86 oF), and use within 4 hours.
Warning: Only licensed healthcare professionals who are qualified by specific training in the safe use and handling of radionuclides should use radiopharmaceuticals.
See Prescribing Information for preparation and administration instructions.
Manufactured for:
Telix Pharmaceuticals (US), Inc.
By:
Grand River Aspetic Manufacturing
140 Front Ave SW
Grand Rapids, MI 49504 USA