NDC Code(s) : 72572-067-01, 72572-067-10
Packager : Civica, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| cisatracurium besylatecisatracurium besylate INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Civica, Inc.(081373942) |
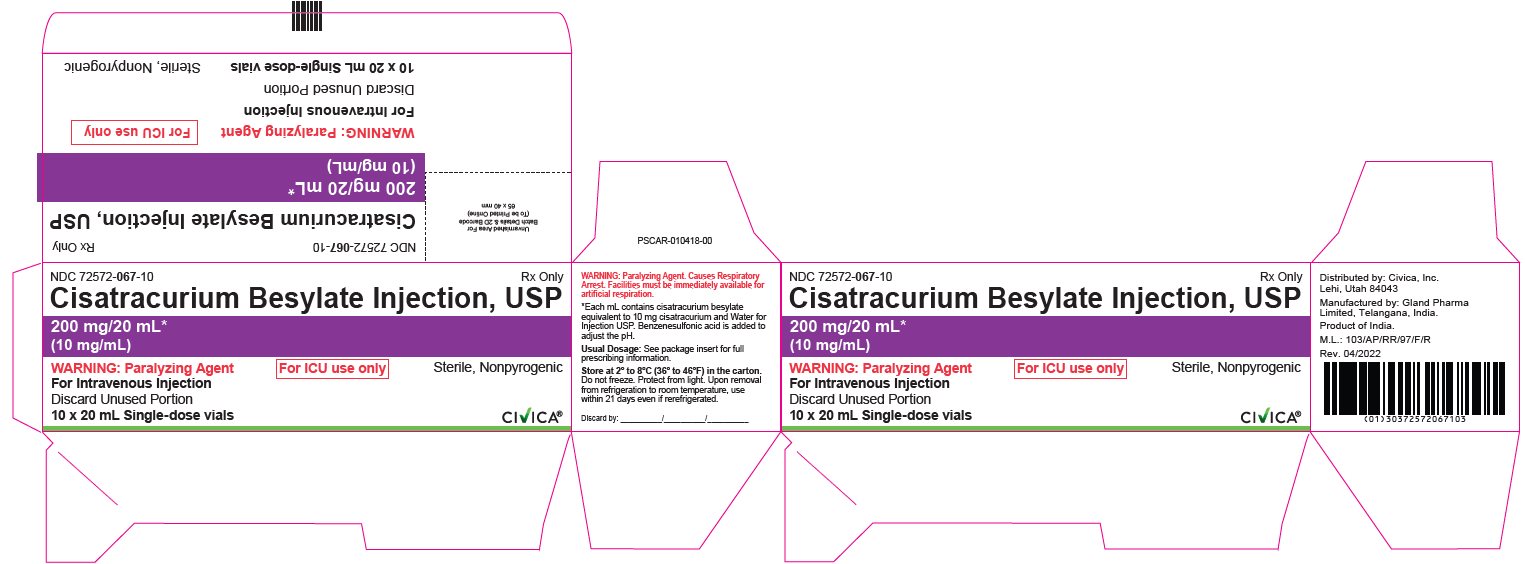
PRINCIPAL DISPLAY PANEL
- NDC 72572-067-10 Rx Only
Cisatracurium Besylate Injection, USP
10 mg/5 mL*
(2 mg/mL)
- WARNING: Paralyzing Agent. For ICU use only Sterile, Nonpyrogenic
For Intravenous Injection
Discard Unused Portion
10 x 20 mL Single-dose vials
CIVICA