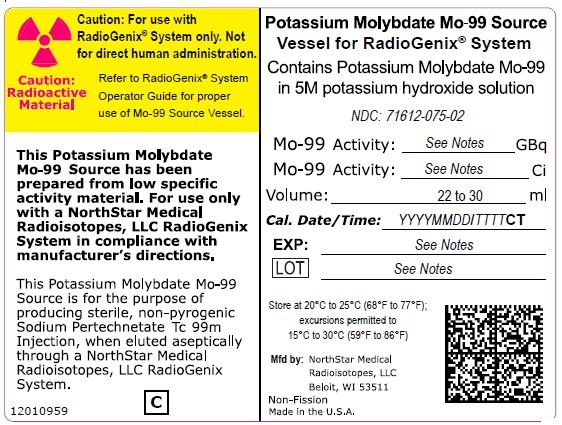
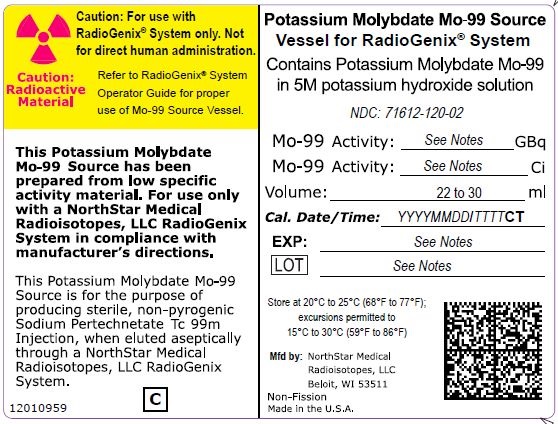
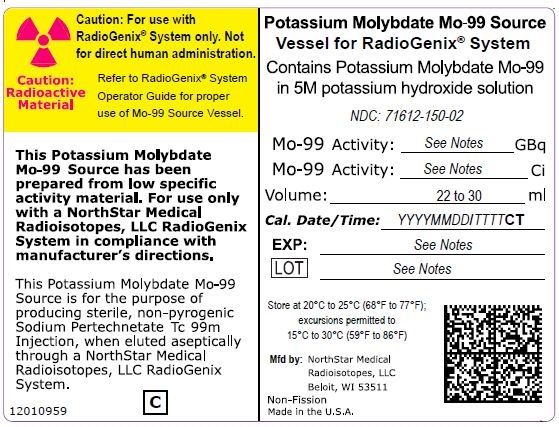
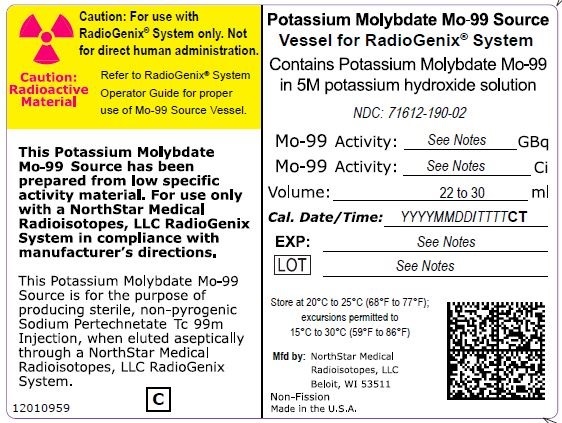
NDC Code(s) : 71612-120-02, 71612-150-02, 71612-190-02, 71612-075-02
Packager : NorthStar Medical Radioisotopes, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| RadioGenix SystemTechnetium Tc 99m Generator INJECTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| RadioGenix SystemTechnetium Tc 99m Generator INJECTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| RadioGenix SystemTechnetium Tc 99m Generator INJECTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| RadioGenix SystemTechnetium Tc 99m Generator INJECTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| LABELER - NorthStar Medical Radioisotopes, LLC(025677872) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| NorthStar Medical Radioisotopes, LLC | 080328423 | api manufacture(71612-075, 71612-120, 71612-150, 71612-190), pack(71612-075, 71612-120, 71612-150, 71612-190), label(71612-075, 71612-120, 71612-150, 71612-190) | |
PRINCIPAL DISPLAY PANEL