NDC Code(s) : 70710-1757-2, 70710-1757-6, 70710-1758-2, 70710-1758-6, 70710-1759-2, 70710-1759-6, 70710-1760-2, 70710-1760-6, 70710-1761-2, 70710-1761-6, 70710-1762-2, 70710-1762-6, 70710-1763-2, 70710-1763-6
Packager : Zydus Pharmaceuticals USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Enoxaparin SodiumEnoxaparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Enoxaparin SodiumEnoxaparin Sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Zydus Pharmaceuticals USA Inc.(156861945) |
| REGISTRANT - Zydus Pharmaceuticals USA Inc.(156861945) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Italfarmaco SpA | 428179329 | MANUFACTURE(70710-1757, 70710-1758, 70710-1759, 70710-1760, 70710-1761, 70710-1762, 70710-1763) | |
PRINCIPAL DISPLAY PANEL
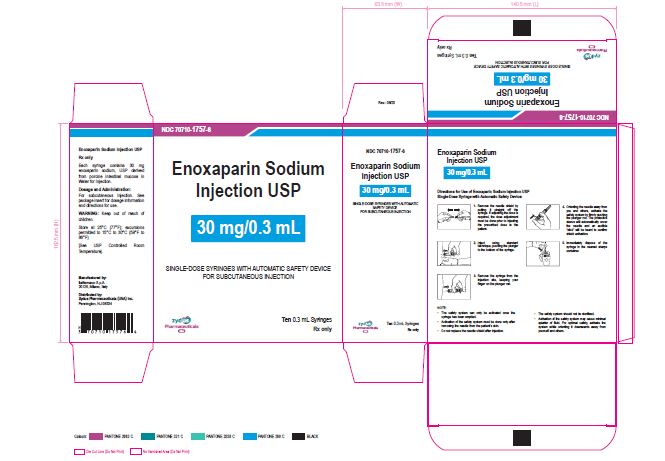
NDC 70710-1757-6
Enoxaparin Sodium Injection USP
30 mg/0.3 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.3 mL Syringes
Zydus
PRINCIPAL DISPLAY PANEL
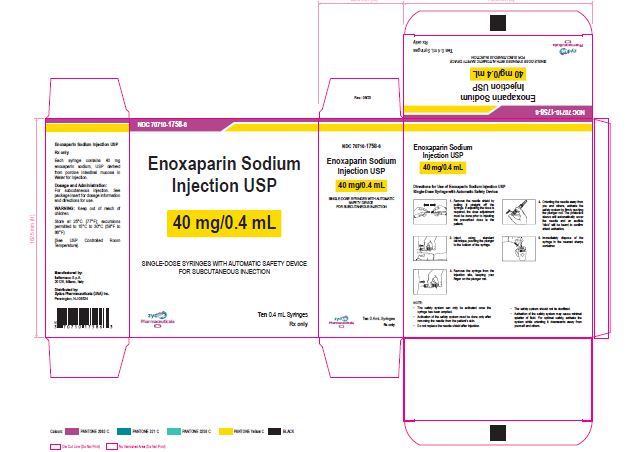
NDC 70710-1758-6
Enoxaparin Sodium Injection USP
40 mg/0.4 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.4 mL Syringes
Zydus
PRINCIPAL DISPLAY PANEL
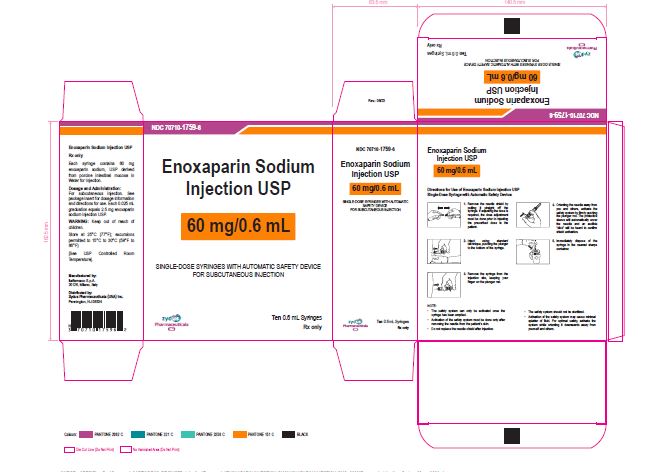
NDC 70710-1759-6
Enoxaparin Sodium Injection USP
60 mg/0.6 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.6 mL Syringes
Zydus
PRINCIPAL DISPLAY PANEL
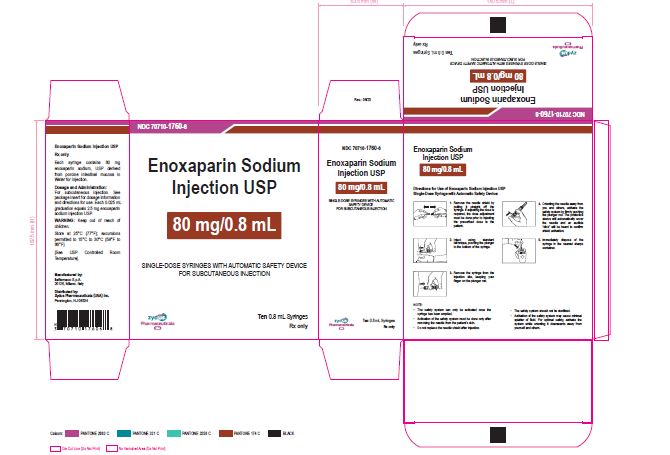
NDC 70710-1760-6
Enoxaparin Sodium Injection USP
80 mg/0.8 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.8 mL Syringes
Zydus
PRINCIPAL DISPLAY PANEL
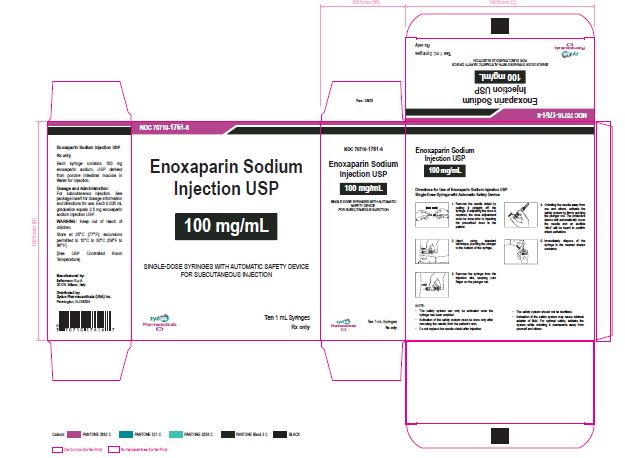
NDC 70710-1761-6
Enoxaparin Sodium Injection USP
100 mg/1 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 1 mL Syringes
Zydus
PRINCIPAL DISPLAY PANEL
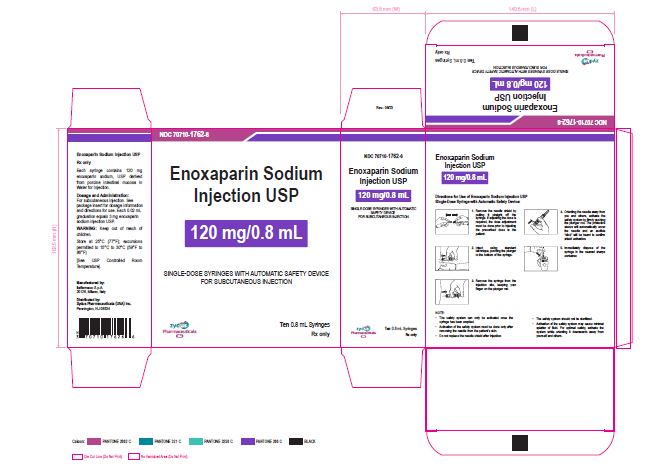
NDC 70710-1762-6
Enoxaparin Sodium Injection USP
120 mg/0.8 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 0.8 mL Syringes
Zydus
PRINCIPAL DISPLAY PANEL
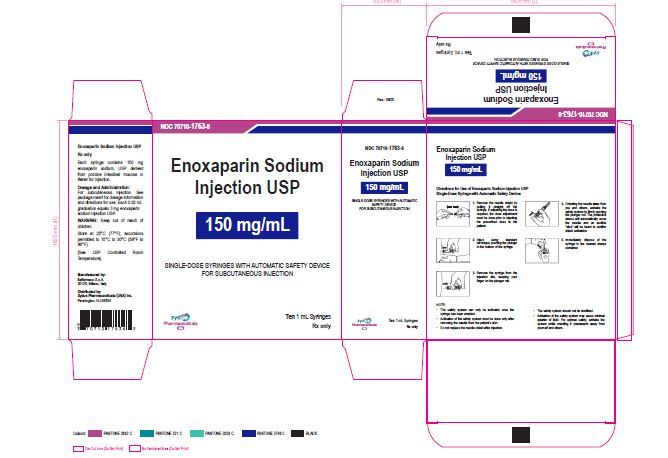
NDC 70710-1763-6
Enoxaparin Sodium Injection USP
150 mg/1 mL
SINGLE-DOSE SYRINGES WITH AUTOMATIC SAFETY DEVICE
FOR SUBCUTANEOUS INJECTION
Ten 1 mL Syringes
Zydus