NDC Code(s) : 70710-1191-1, 70710-1191-8, 70710-1192-1, 70710-1192-8, 70710-1193-1, 70710-1193-8, 70710-1194-1, 70710-1194-8, 70710-1195-1, 70710-1195-8
Packager : Zydus Pharmaceuticals USA Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| EstradiolEstradiol PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| EstradiolEstradiol PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| EstradiolEstradiol PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| EstradiolEstradiol PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| EstradiolEstradiol PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Zydus Pharmaceuticals USA Inc.(156861945) |
| REGISTRANT - ZYDUS NOVELTECH INC, USA(801012530) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Zydus Lifesciences Limited | 918596198 | ANALYSIS(70710-1191, 70710-1192, 70710-1193, 70710-1194, 70710-1195), MANUFACTURE(70710-1191, 70710-1192, 70710-1193, 70710-1194, 70710-1195) | |
PRINCIPAL DISPLAY PANEL
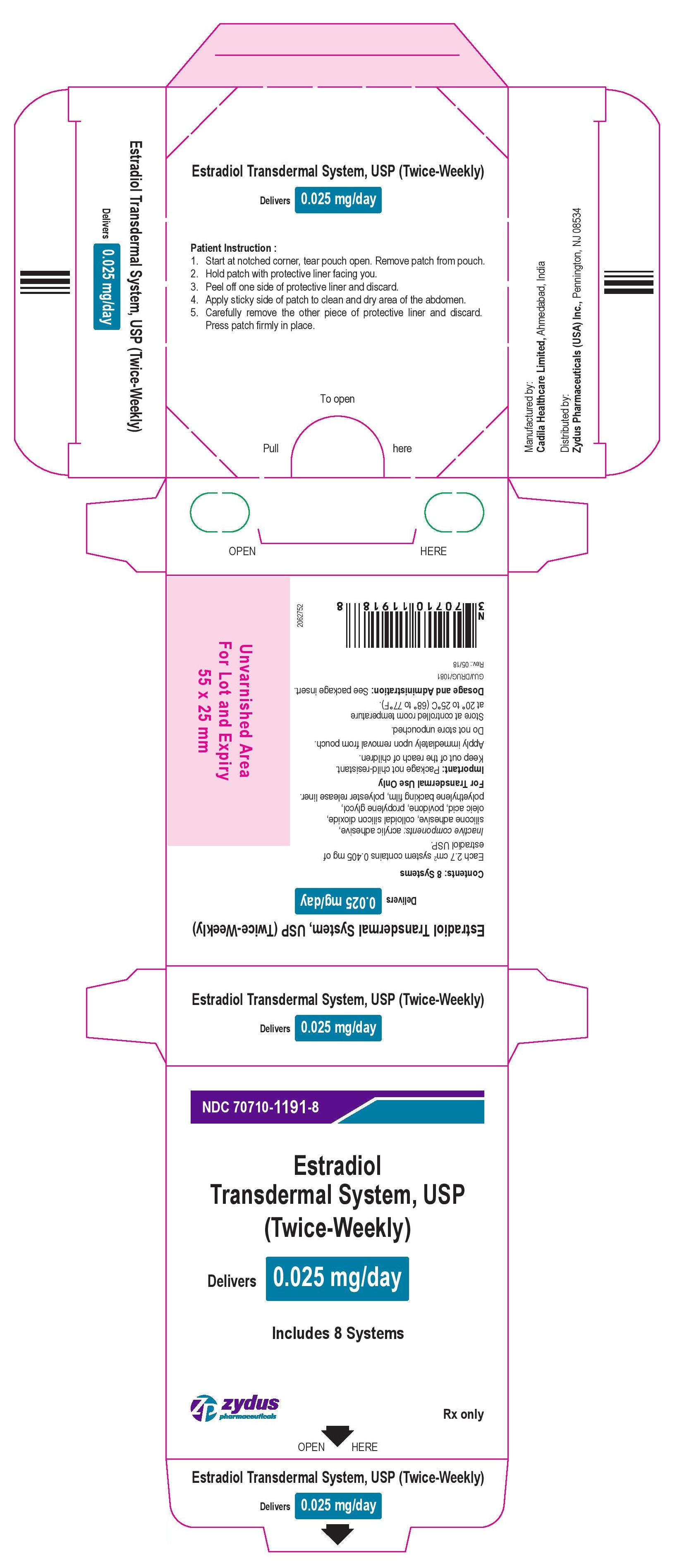
Package Label – 0.025 mg
NDC 70710-1191-8
Estradiol Transdermal System, USP
(Twice-Weekly)
Delivers 0.025 mg/day
Includes 8 Systems
Rx Only
zydus pharmaceuticals
PRINCIPAL DISPLAY PANEL
Package Label – 0.0375 mg
NDC 70710-1192-8
Estradiol Transdermal System, USP
(Twice-Weekly)
Delivers 0.0375 mg/day
Includes 8 Systems
Rx Only
zydus pharmaceuticals

PRINCIPAL DISPLAY PANEL
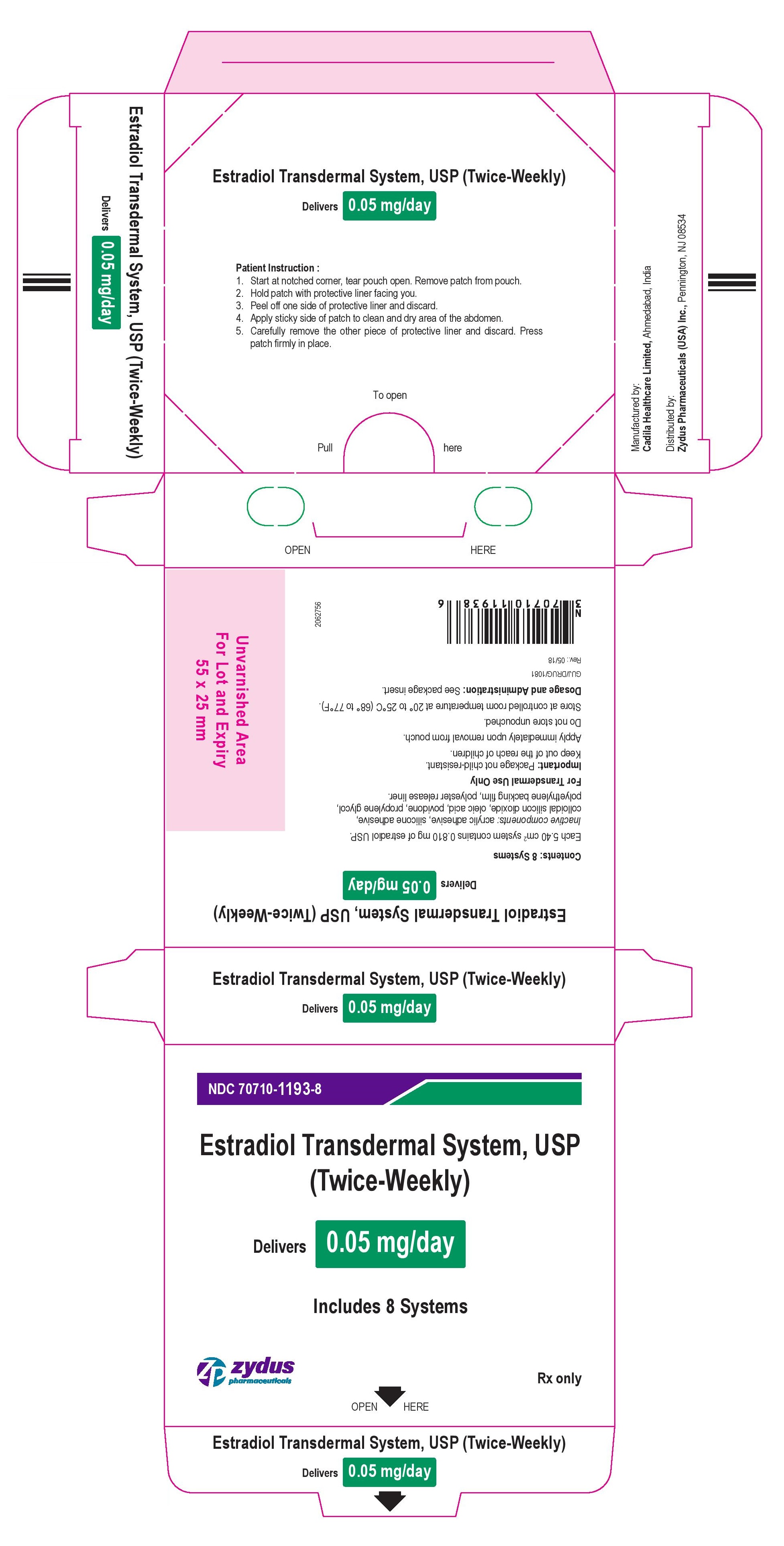
Package Label – 0.05 mg
NDC 70710-1193-8
Estradiol Transdermal System, USP
(Twice-Weekly)
Delivers 0.05 mg/day
Includes 8 Systems
Rx Only
zydus pharmaceuticals
PRINCIPAL DISPLAY PANEL
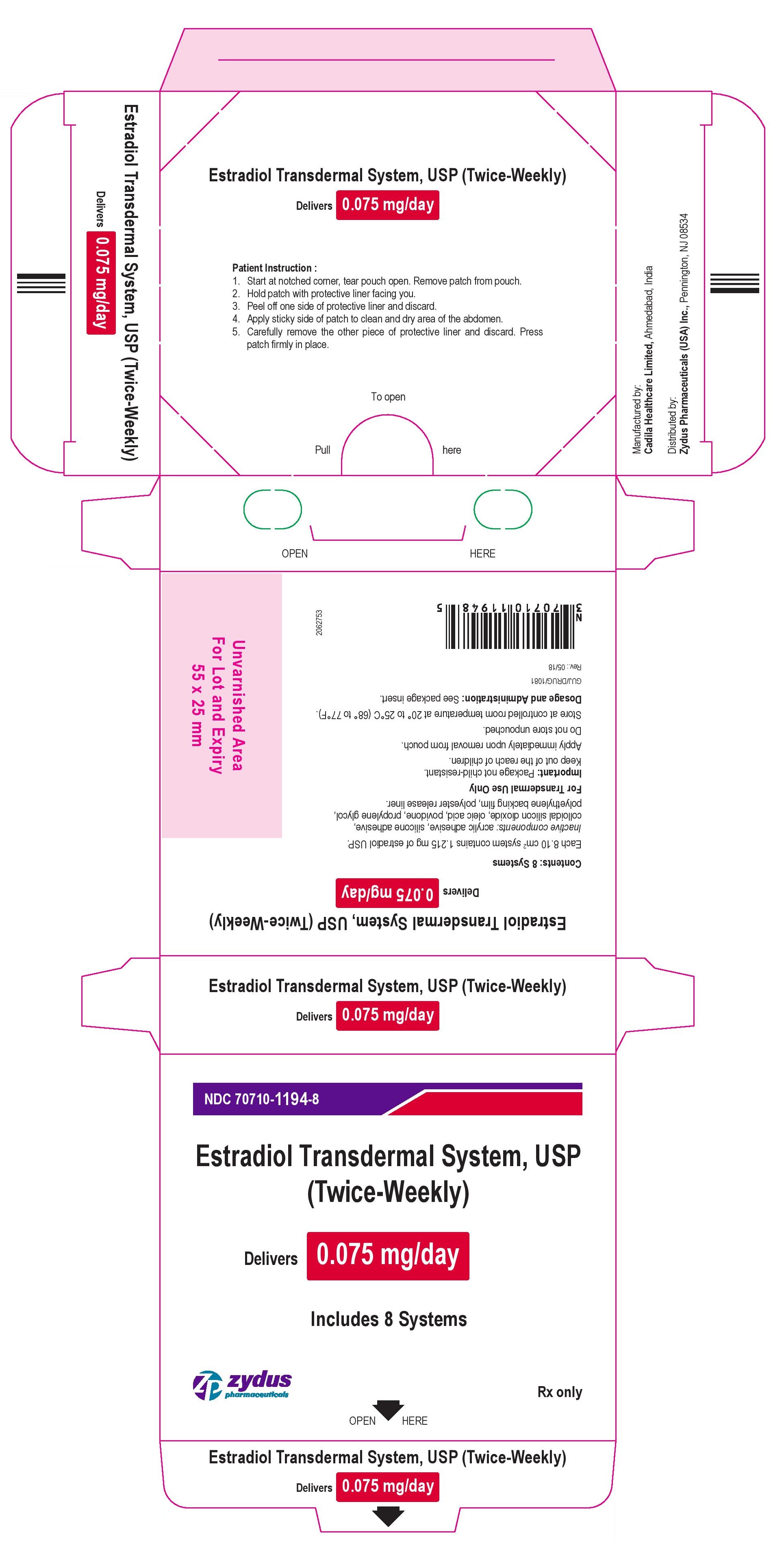
Package Label – 0.075 mg
NDC 70710-1194-8
Estradiol Transdermal System, USP
(Twice-Weekly)
Delivers 0.075 mg/day
Includes 8 Systems
Rx Only
zydus pharmaceuticals
PRINCIPAL DISPLAY PANEL
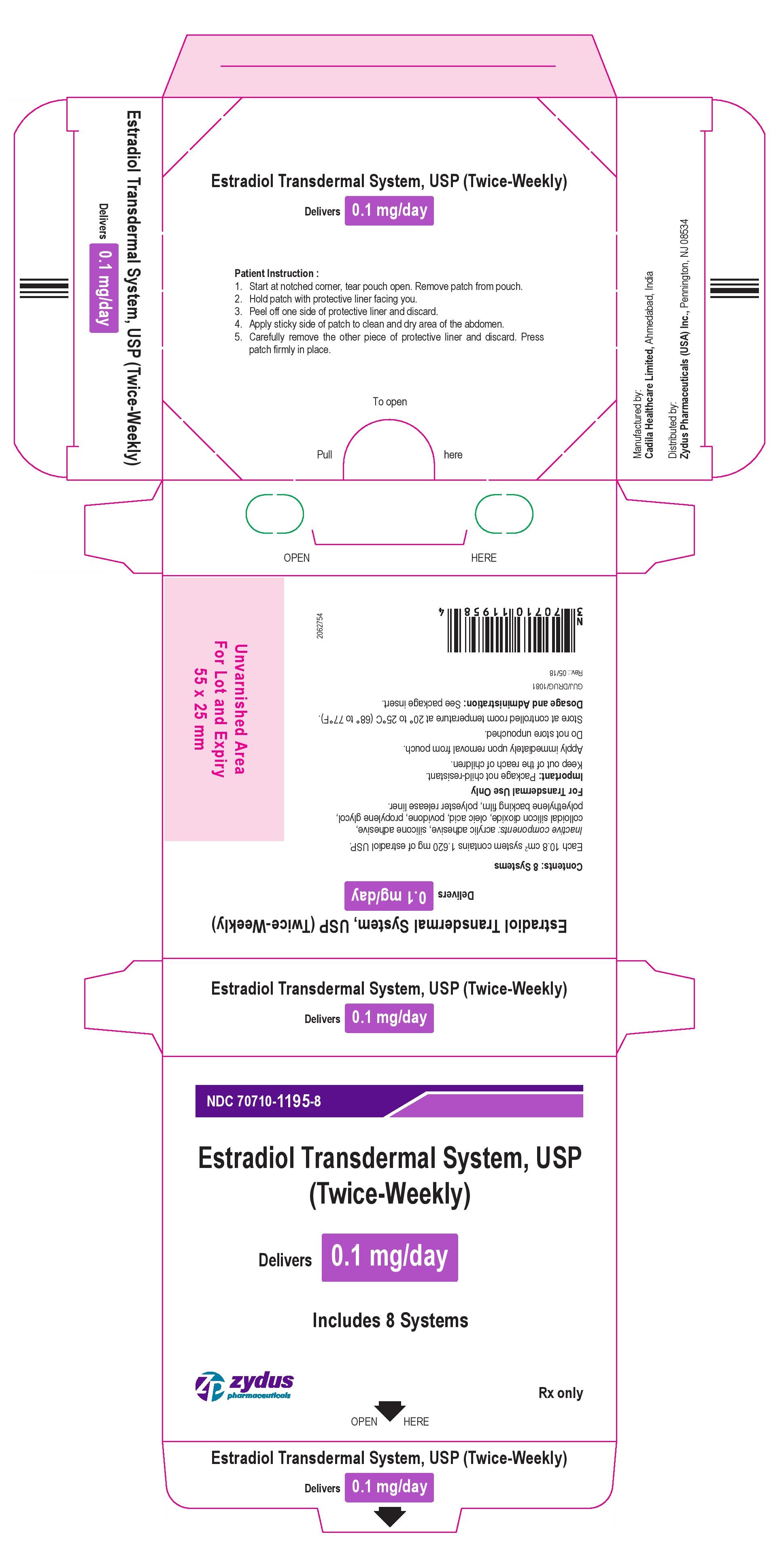
Package Label – 0.1 mg
NDC 70710-1195-8
Estradiol Transdermal System, USP
(Twice-Weekly)
Delivers 0.1 mg/day
Includes 8 Systems
Rx Only
zydus pharmaceuticals