NDC Code(s) : 68968-0514-8, 68968-0525-8
Packager : Noven Therapeutics, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CombiPatch (estradiol/norethindrone acetate transdermal system)estradiol/norethindrone acetate transdermal system PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| CombiPatch (estradiol/norethindrone acetate transdermal system)estradiol/norethindrone acetate transdermal system PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Noven Therapeutics, LLC(166888268) |
| REGISTRANT - Noven Pharmaceuticals, Inc.(148585441) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Noven Pharmaceuticals, Inc. | 148585441 | analysis(68968-0514, 68968-0525), manufacture(68968-0514, 68968-0525) | |
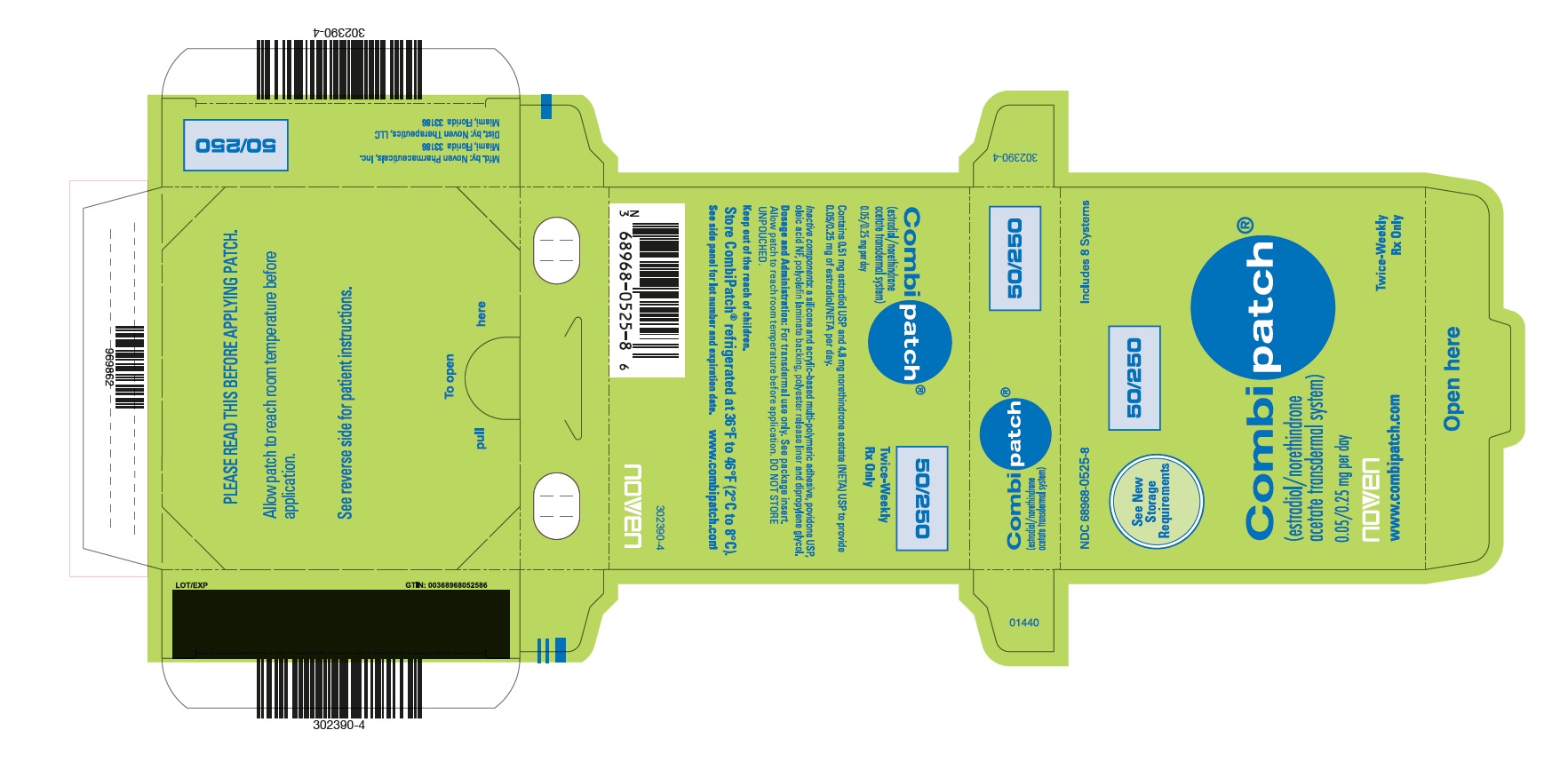
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - NDC - 68968-0514-8 CombiPatch Count - 50/140
NDC 68968-0514-8
Includes 8 Systems
See New Storage Requirements
Combipatch ® 50/140
(estradiol/norethindrone acetate transdermal system)
0.05/0.14 mg per day
Noven
www.combipatch.com
Twice-Weekly Rx Only
Combipatch® 50/140
(estradiol/norethindrone acetate transdermal system)
0.05/0.14 mg per day
Twice-Weekly Rx Only
Contains 0.62 mg estradiol USP and 2.7 mg norethindrone acetate (NETA) USP to provide 0.05/0.14 mg of estradiol/NETA per day.
Inactive components: a silicone and acrylic-based multi-polymeric adhesive, povidone USP, oleic acid NF, polyolefin laminate backing, polyester release liner and dipropylene glycol.
Dosage and Administration: For transdermal use only. See package insert. Allow patch to reach room temperature before application. DO NOT STORE UNPOUCHED.
Keep out of the reach of children.
Store Combipatch® refrigerated at 36°F to 46°F (2°C to 8°C)
See side panel for lot number and expiration date. www.combipatch.com
N3 68968-0514-8 0 302391-4
Noven
PLEASE READ THIS BEFORE APPLYING PATCH.
Allow patch to reach room temperature before application.
See reverse side for patient instructions.
pull To open here
LOT/EXP GTIN 00368968051480
969864
Mfd. By: Noven Pharmaceuticals, Inc.
Miami, Florida 33186
Dist. By Noven Therapeutics, LLC
Miami, Florida 33186
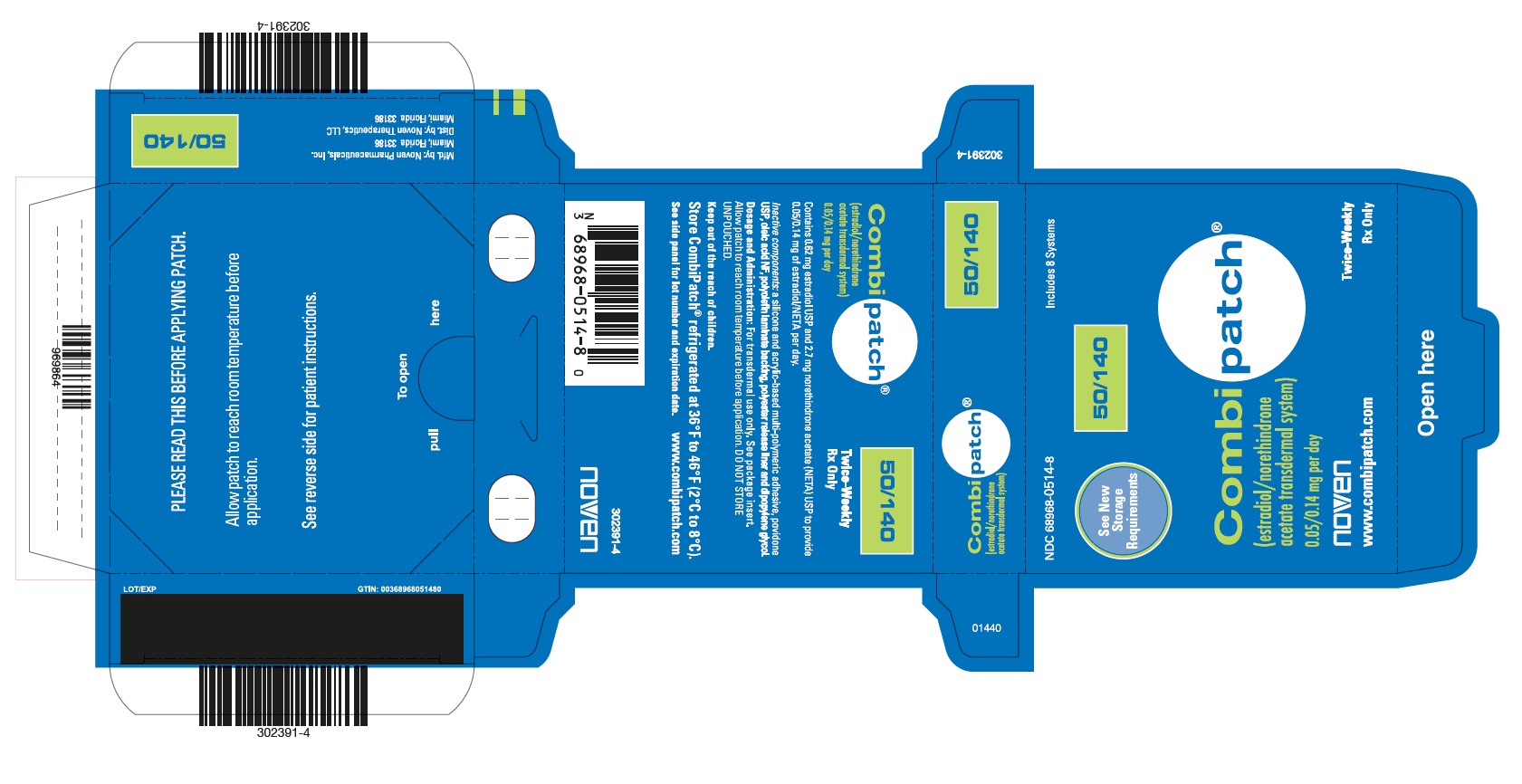
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - NDC - 68968-0525-8 CombiPatch Count - 50/250
NDC 68968-0525-8
Includes 8 Systems
See New Storage Requirements
Combipatch® 50/250
(estradiol/norethindrone acetate transdermal system)
0.05/0.25 mg per day
Noven
www.combipatch.com
Twice-Weekly Rx Only
Combipatch® 50/250
(estradiol/norethindrone acetate transdermal system)
0.05/0.25 mg per day
Twice-Weekly Rx Only
Contains 0.51 mg estradiol USP and 4.8 mg norethindrone acetate (NETA) USP to provide 0.05/0.25 mg of estradiol/NETA per day.
Inactive components: a silicone and acrylic-based multi-polymeric adhesive, povidone USP, oleic acid NF, polyolefin laminate backing, polyester release liner and dipropylene glycol.
Dosage and Administration: For transdermal use only. See package insert. Allow patch to reach room temperature before application. DO NOT STORE UNPOUCHED.
Keep out of the reach of children.
Store Combipatch® refrigerated at 36°F to 46°F (2°C to 8°C)
See side panel for lot number and expiration date. www.combipatch.com
N3 68968-0525-8 0 302390-4
Noven
PLEASE READ THIS BEFORE APPLYING PATCH.
Allow patch to reach room temperature before application.
See reverse side for patient instructions.
pull To open here
LOT/EXP GTIN 00368968052586
969864
Mfd. By: Noven Pharmaceuticals, Inc.
Miami, Florida 33186
Dist. By Noven Therapeutics, LLC
Miami, Florida 33186