NDC Code(s) : 68180-838-71, 68180-838-73
Packager : Lupin Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Norgestimate and ethinyl estradiol Norgestimate and ethinyl estradiol KIT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Lupin Pharmaceuticals, Inc.(089153071) |
| REGISTRANT - LUPIN LIMITED(675923163) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LUPIN LIMITED | 650582310 | MANUFACTURE(68180-838), PACK(68180-838) | |
PRINCIPAL DISPLAY PANEL
Carton Label
NDC 68180-838-73
Norgestimate and Ethinyl Estradiol Tablets USP, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg and 0.25 mg/0.035 mg
Blister pack containing 28 tablets packed in a Pouch. Such three Pouches are packaged in a Carton.
Rx only
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. THIS PACKAGE IS NOT CHILD RESISTANT.
Pharmacist: Each unit contains information intended for the patients. This informational piece is to be provided to the patient with each prescription.

Pouch Label
NDC 68180-838-71
Norgestimate and Ethinyl Estradiol Tablets USP, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg and 0.25 mg/0.035
Blister pack containing 28 tablets packed in a Pouch.
Rx only
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. THIS PACKAGE IS NOT CHILD RESISTANT.
Pharmacist: Each unit contains information intended for the patients. This informational piece is to be provided to the patient with each prescription.

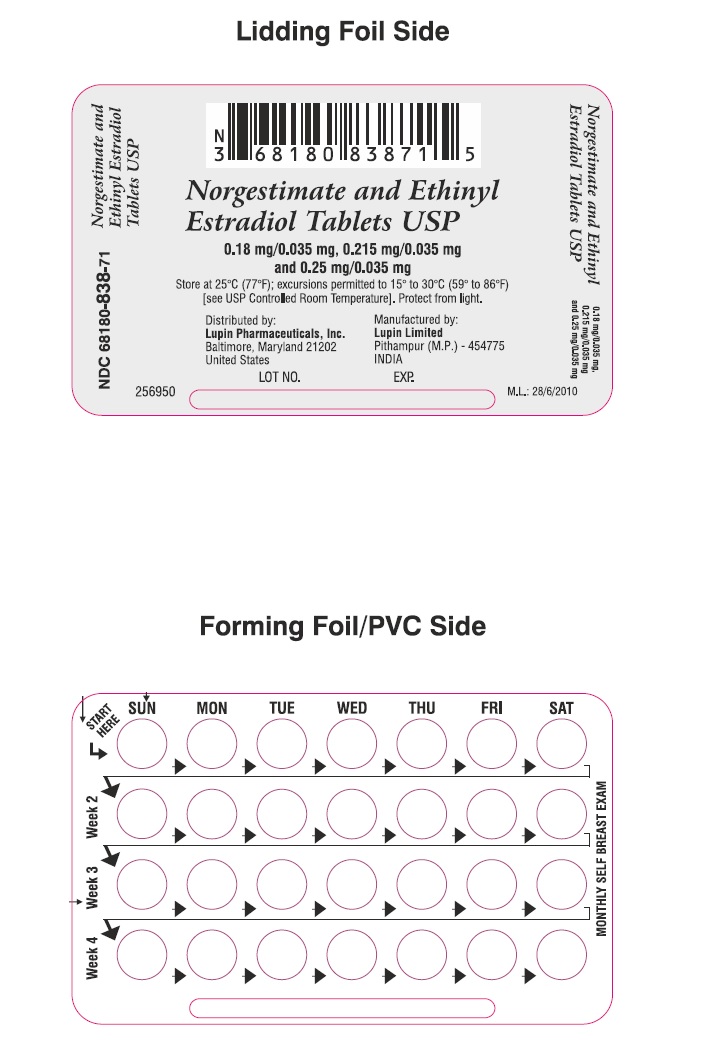
Blister Label
NDC 68180-838-71
Norgestimate and Ethinyl Estradiol Tablets USP, 0.18 mg/0.035 mg, 0.215 mg/0.035 mg and 0.25 mg/0.035
28 Tablets
Rx only
KEEP THIS AND ALL MEDICATION OUT OF THE REACH OF CHILDREN. THIS PACKAGE IS NOT CHILD RESISTANT.
Pharmacist: Each unit contains information intended for the patients. This informational piece is to be provided to the patient with each prescription.