NDC Code(s) : 67877-638-30, 67877-638-90, 67877-638-05, 67877-638-33, 67877-638-38, 67877-639-30, 67877-639-90, 67877-639-05, 67877-639-33, 67877-639-38, 67877-640-30, 67877-640-90, 67877-640-05, 67877-640-33, 67877-640-38, 67877-641-30, 67877-641-90, 67877-641-05, 67877-641-33, 67877-641-38, 67877-642-30, 67877-642-90, 67877-642-05, 67877-642-33, 67877-642-38
Packager : Ascend Laboratories, LLC
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| lurasidone hydrochloride lurasidone hydrochloride TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| lurasidone hydrochloride lurasidone hydrochloride TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| lurasidone hydrochloride lurasidone hydrochloride TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| lurasidone hydrochloride lurasidone hydrochloride TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| lurasidone hydrochloride lurasidone hydrochloride TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| LABELER - Ascend Laboratories, LLC(141250469) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Alkem Laboratories Limited | 677605851 | ANALYSIS(67877-638, 67877-639, 67877-640, 67877-641, 67877-642), MANUFACTURE(67877-638, 67877-639, 67877-640, 67877-641, 67877-642), PACK(67877-638, 67877-639, 67877-640, 67877-641, 67877-642) | |
PRINCIPAL DISPLAY PANEL
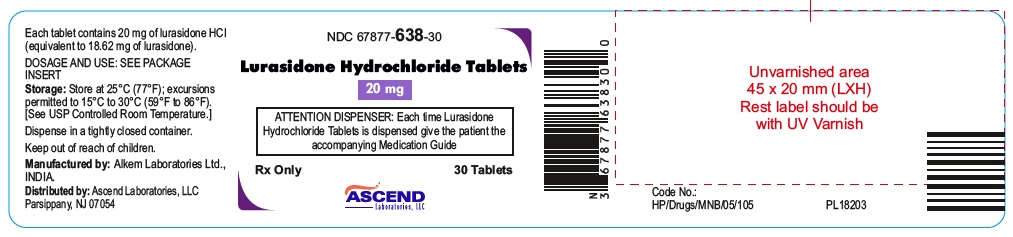
NDC: 67877-638-30
Lurasidone Hydrochloride Tablets 20 mg
30 Tablets
NDC: 67877-639-90
Lurasidone Hydrochloride Tablets 40 mg
90 Tablets

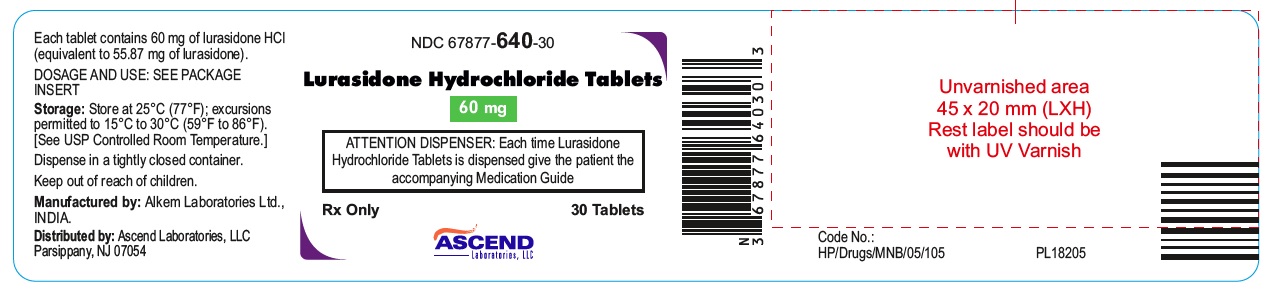
NDC: 67877-640-30
Lurasidone Hydrochloride Tablets 60 mg
30 Tablets
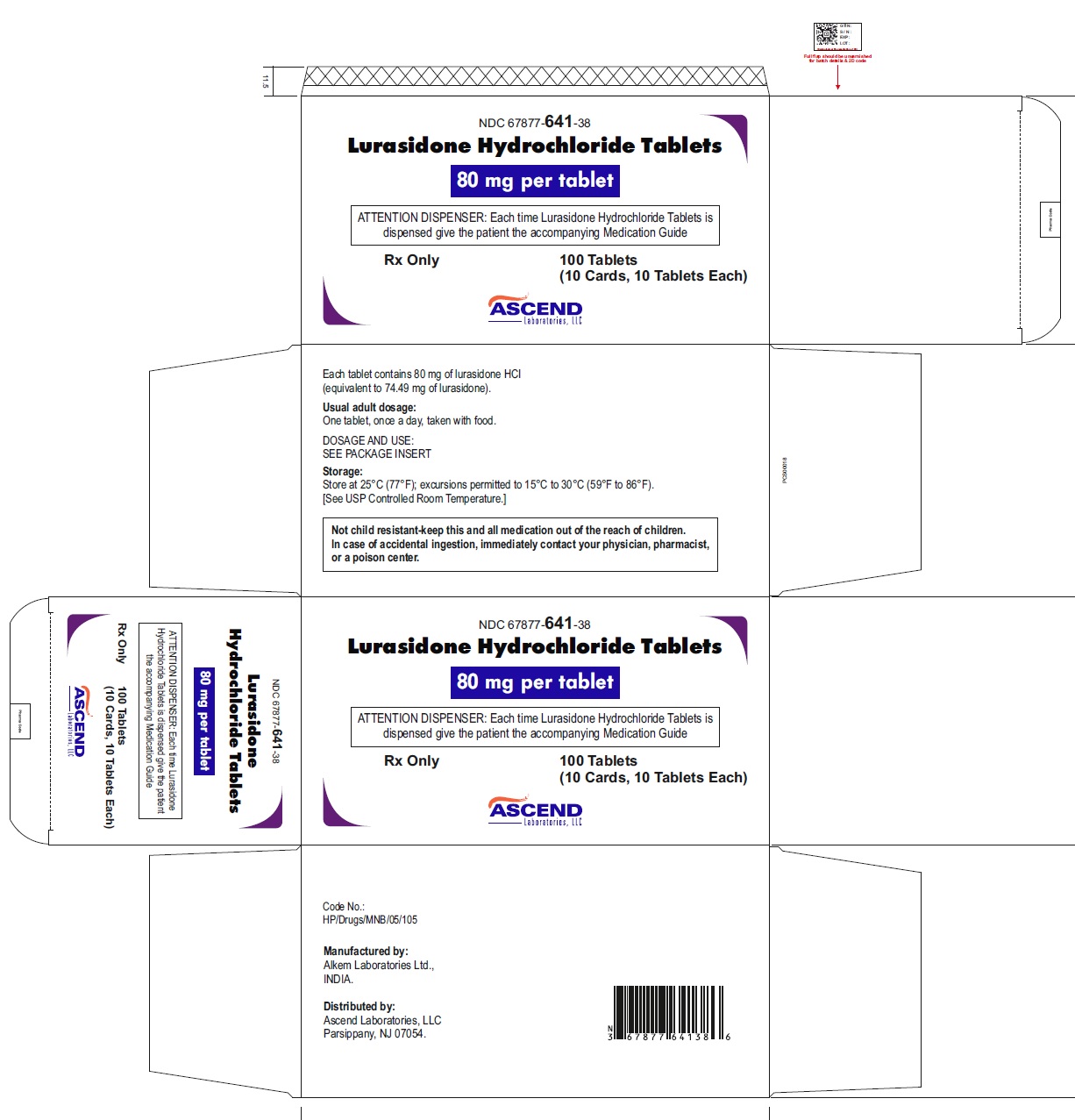
NDC: 67877-641-38
Lurasidone Hydrochloride Tablets 80 mg
Carton of 100 (10X10) Unit dose Tablets
NDC: 67877-642-05
Lurasidone Hydrochloride Tablets 120 mg
500 Tablets










