NDC Code(s) : 67546-212-21, 67546-111-12, 67546-111-14
Packager : Romark Laboratories, L.C.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Alinianitazoxanide POWDER, FOR SUSPENSION | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Alinianitazoxanide TABLET | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Romark Laboratories, L.C.(877685123) |
PRINCIPAL DISPLAY PANEL
NDC 67546-111-12
Alinia®
(nitazoxanide) tablets
500 mg
Rx Only 30 TABLETS

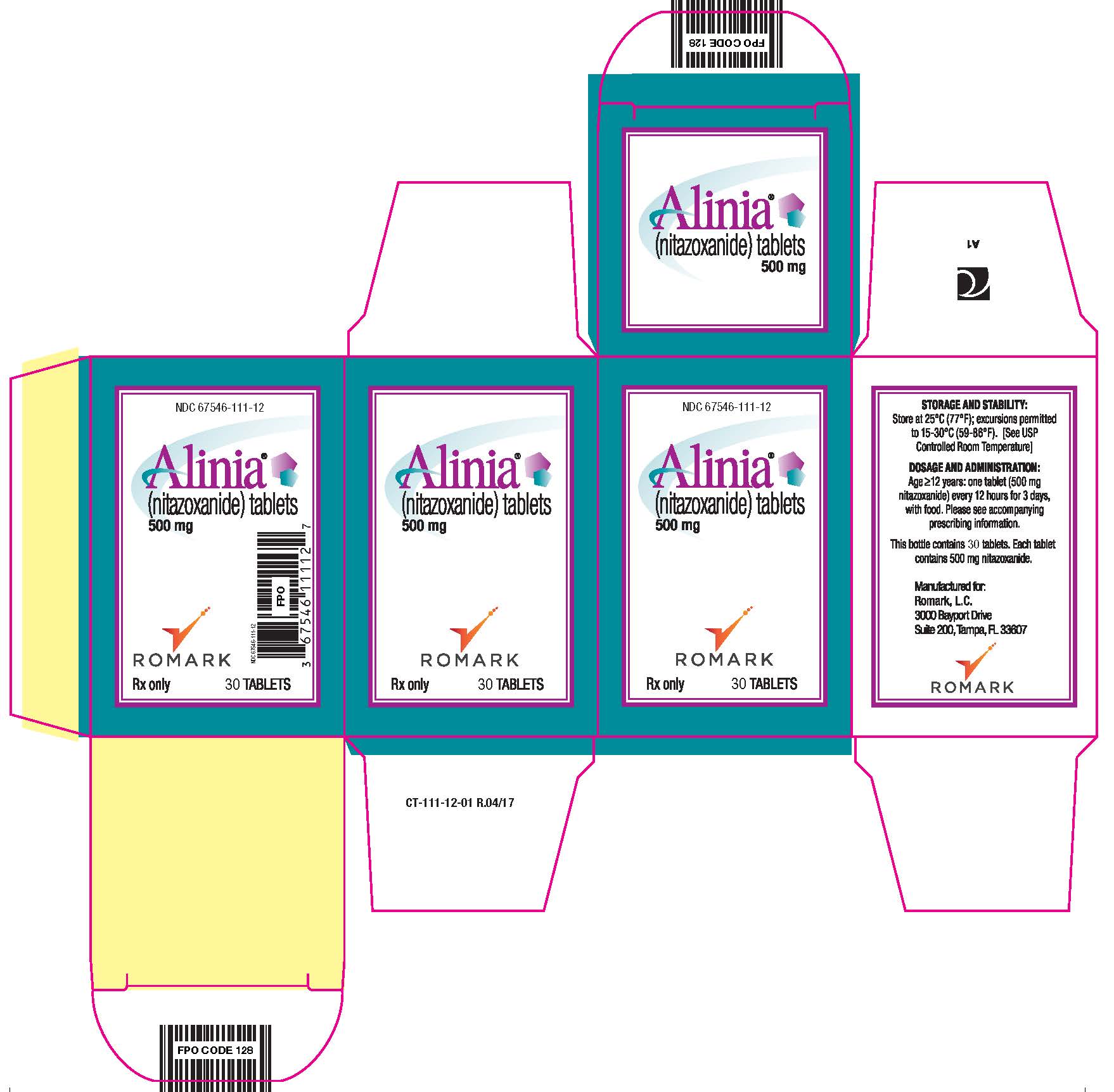
PRINCIPAL DISPLAY PANEL
NDC 67546-111-12
Alinia (nitazoxanide) tablets
500 mg
Rx Only 30 TABLETS
PRINCIPAL DISPLAY PANEL
NDC 67546-111-17
Alinia®
(nitazoxanide) tablets
500 mg
Rx only 2 TABLETS SAMPLE

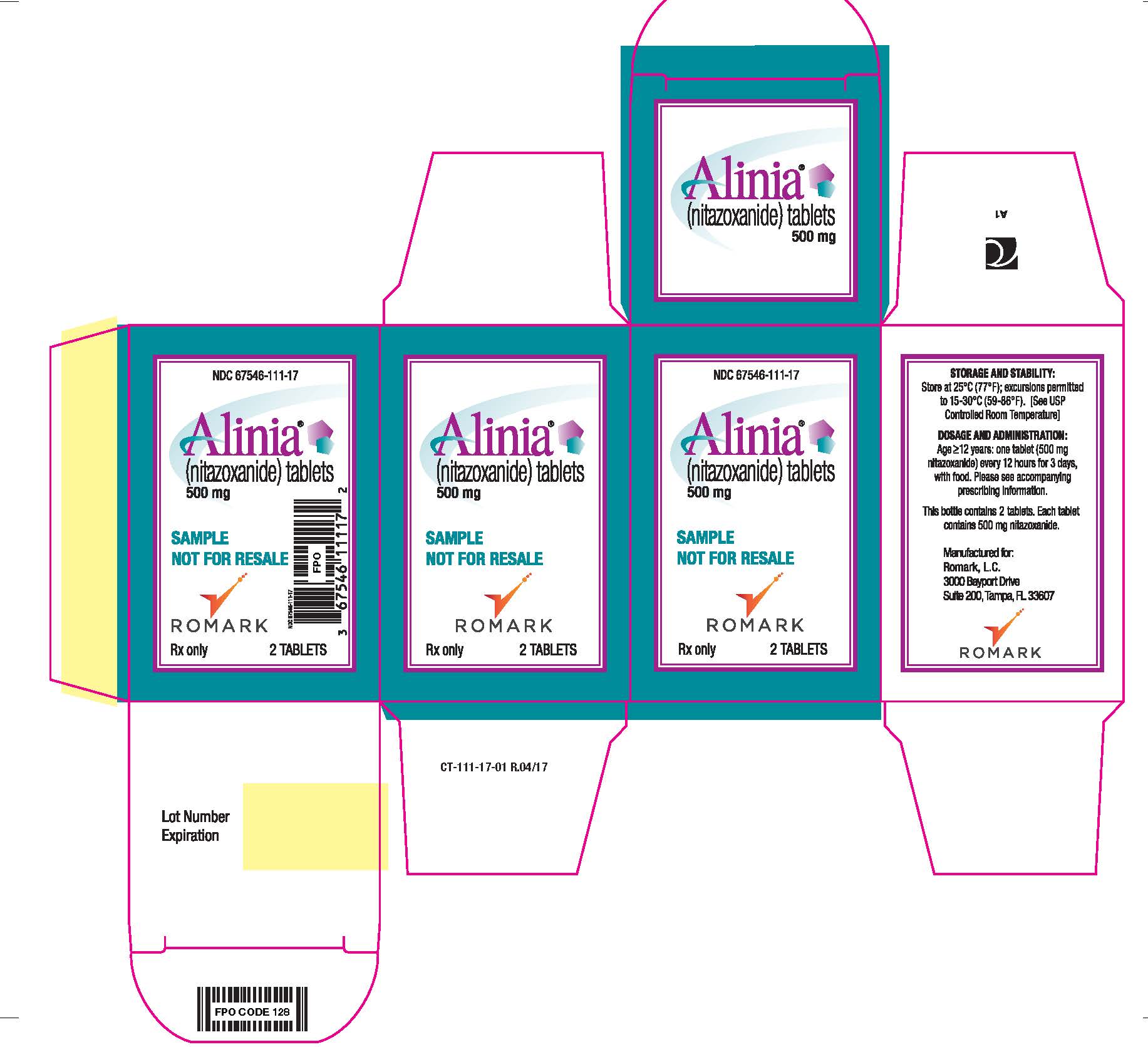
PRINCIPAL DISPLAY PANEL
NDC 67546-111-17
Alinia (nitazoxanide) tablets
500 mg
SAMPLE NOT FOR RESALE
Romark logo
Rx Only 2 TABLETS
PRINCIPAL DISPLAY PANEL
NDC 67546-111-14
Alinia® (nitazoxinde) tablets
500 mg
Rx only 12 TABLETS

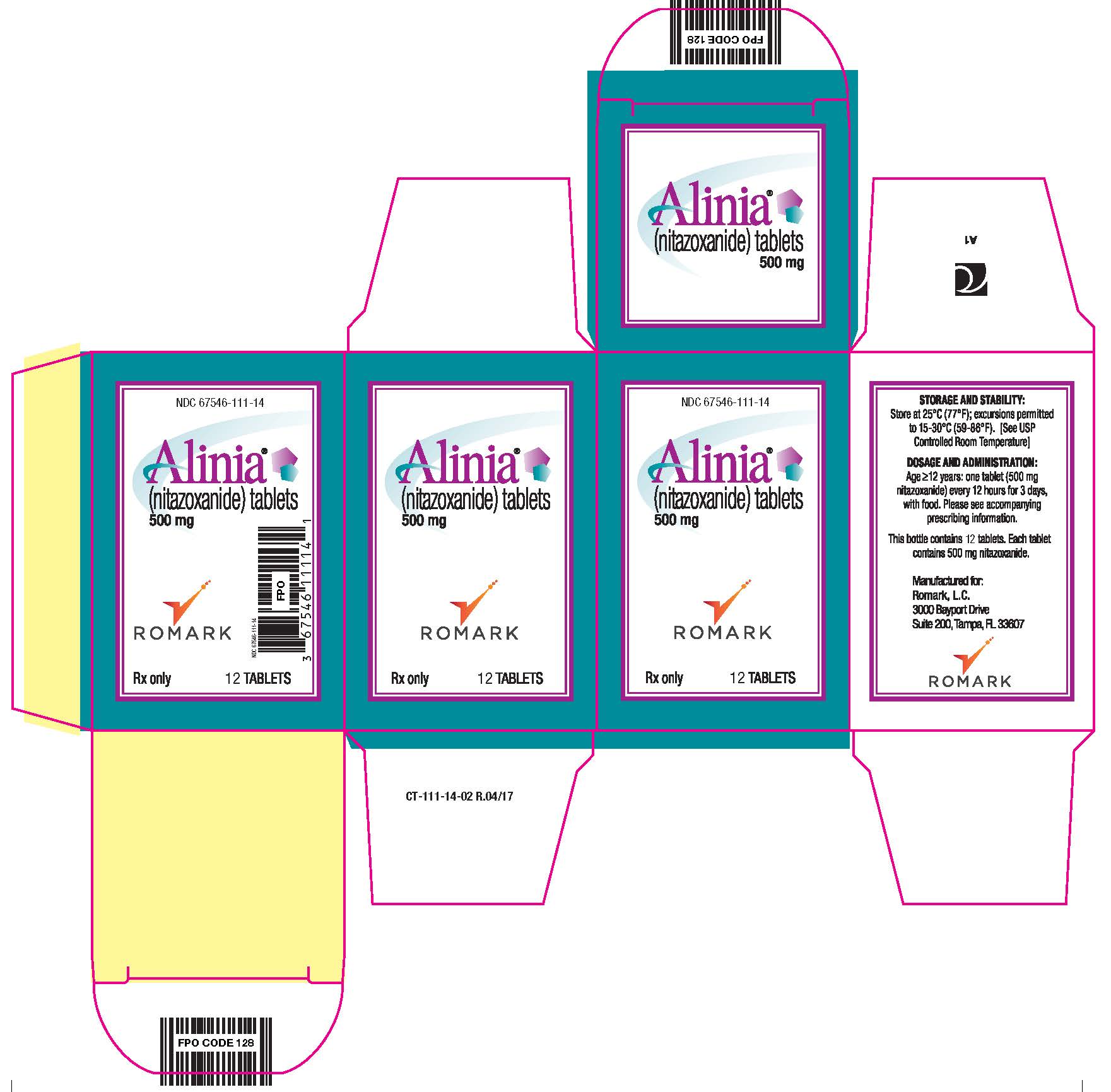
PRINCIPAL DISPLAY PANEL
NDC 67546-111-14
Alinia®
(nitazoxanide) tablets
500 mg
Romark Logo
Rx only 12 TABLETS
PRINCIPAL DISPLAY PANEL
NDC 67546-212-21
Alinia (nitazoxanide) for Oral Suspension 100 mg/5 mL
Reconstitute with 48 mL water
SHAKE WELL BEFORE USING
Any unused protion must be discarded 7 days after mixing
60 mL reconstituted
Rx only

PRINCIPAL DISPLAY PANEL
 NDC 67546-212-21
NDC 67546-212-21
Alinia (nitazoxanide) for Oral Suspension 100 mg/5 mL
Reconstitute with 48 mL water
SHAKE WELL BEFORE USING
Any unused portion must be discarded 7 days after mixing
60 mL
Rx only









