NDC Code(s) : 67457-778-00, 67457-778-05, 67457-779-30
Packager : Mylan Institutional LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Sulfamethoxazole and Trimethoprimsulfamethoxazole and trimethoprim INJECTION | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Sulfamethoxazole and Trimethoprimsulfamethoxazole and trimethoprim INJECTION | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LABELER - Mylan Institutional LLC(790384502) |
| REGISTRANT - Mylan Pharmaceuticals Inc(059295980) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Mylan Laboratories Limited | 677605290 | MANUFACTURE(67457-778, 67457-779), ANALYSIS(67457-778, 67457-779), STERILIZE(67457-778, 67457-779), PACK(67457-778, 67457-779), LABEL(67457-778, 67457-779) | |
PRINCIPAL DISPLAY PANEL
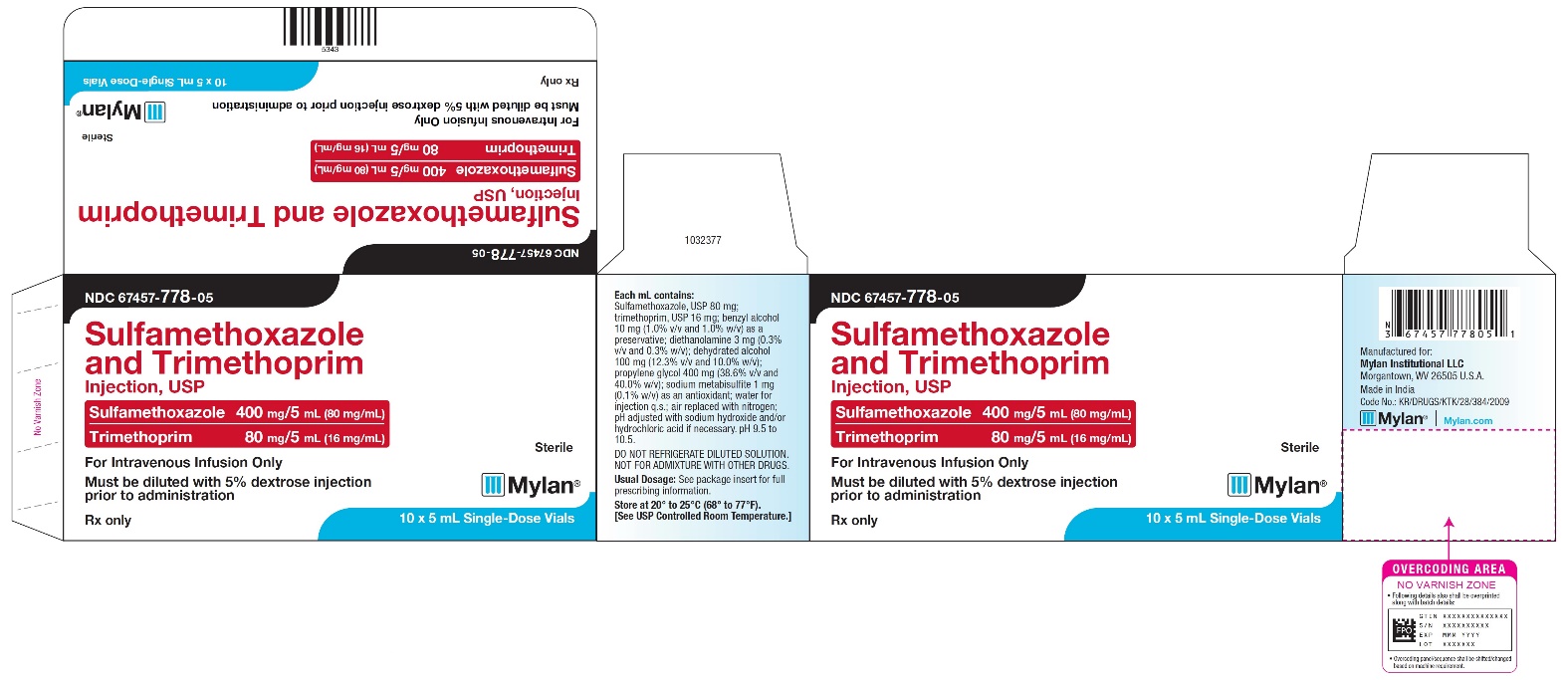
NDC 67457-778-05
Sulfamethoxazole and Trimethoprim Injection, USP
Sulfamethoxazole 400 mg/5mL (80 mg/mL)
Trimethoprim 80 mg/5 mL (16 mg/mL)
For Intravenous Infusion Only
Must be diluted with 5% dextrose injection prior to administration
Sterile
Mylan
Rx only
10 x 5 mL Single-Dose Vials
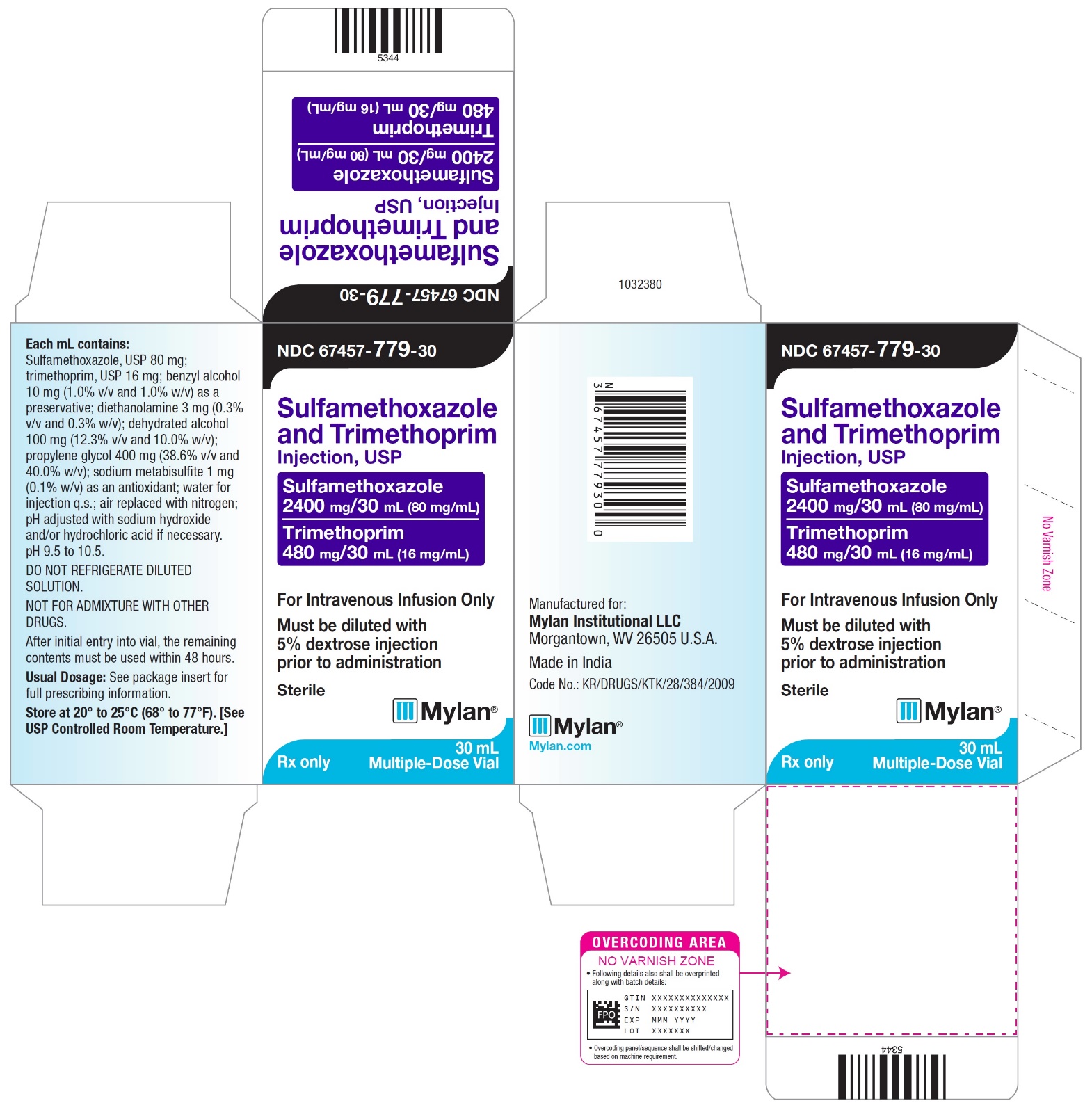
PRINCIPAL DISPLAY PANEL
NDC 67457-779-30
Sulfamethoxazole and Trimethoprim Injection, USP
Sulfamethoxazole 400 mg/5mL (80 mg/mL)
Trimethoprim 80 mg/5 mL (16 mg/mL)
For Intravenous Infusion Only
Must be diluted with 5% dextrose injection prior to administration
Sterile
Mylan
Rx only
30 mL Multiple-Dose Vials