NDC Code(s) : 67253-580-42, 67253-580-43, 67253-580-44, 67253-580-45, 67253-580-46, 67253-580-47, 67253-580-48
Packager : Par Pharmaceutical
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Rheumatrex Methotrexate TABLET | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
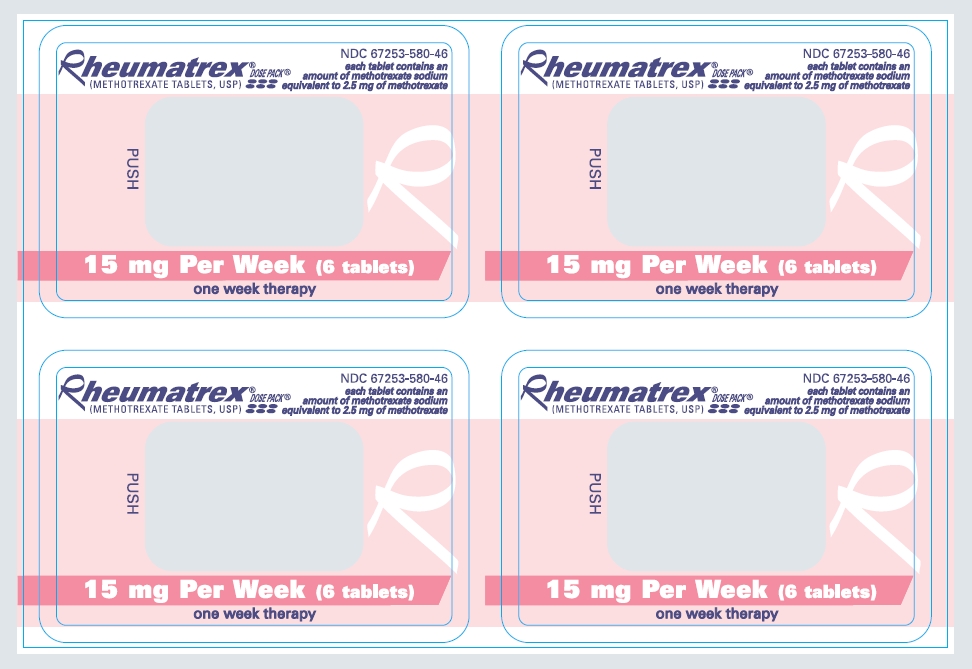
Rheumatrex® DOSE PACK®
(METHOTREXATE TABLETS, USP)
NDC 67253-580-46
each tablet contains an amount of methotrexate sodium equivalent to 2.5 mg of methotrexate
15 mg Per Week (6 tablets)
one week therapy
PRINCIPAL DISPLAY PANEL
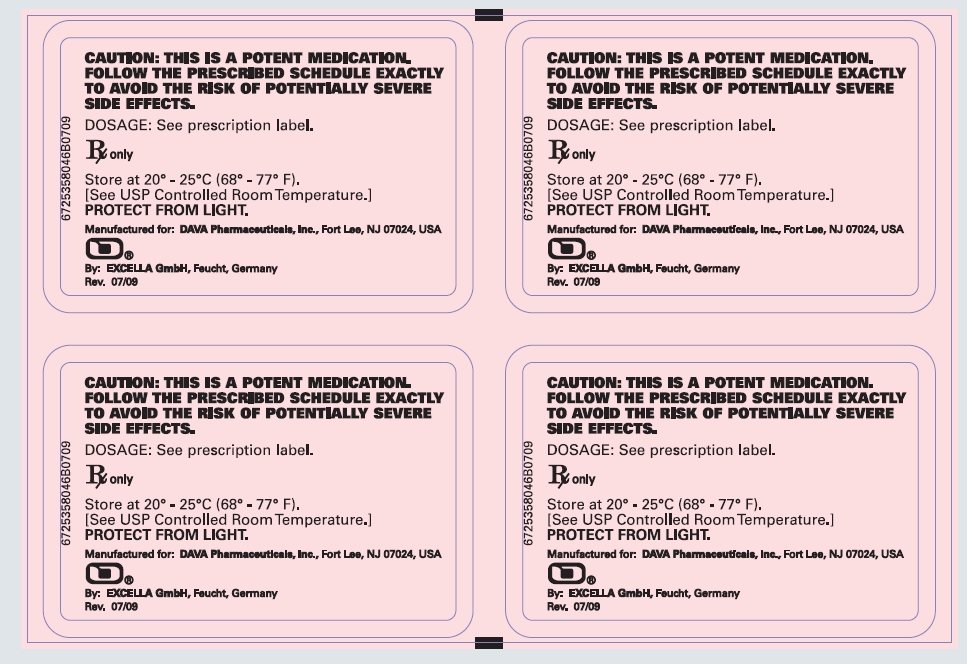
CAUTION: THIS IS A POTENT MEDICATION. FOLLOW THE PRESCRIBED SCHEDULE EXACTLY TO AVOID THE RISK OF POTENTIALLY SEVERE SIDE EFFECTS.
DOSAGE: See prescription label.
Rx only
Store at 20° - 25°C (68° - 77°F).
[See USP Controlled Room Temperature.]
PROTECT FROM LIGHT.
Manufactured for: DAVA Pharmaceuticals, Inc., Fort Lee, NJ 07024, USA
By: Excella GmbH, Feucht, Germany
Rev. 07/09
PRINCIPAL DISPLAY PANEL
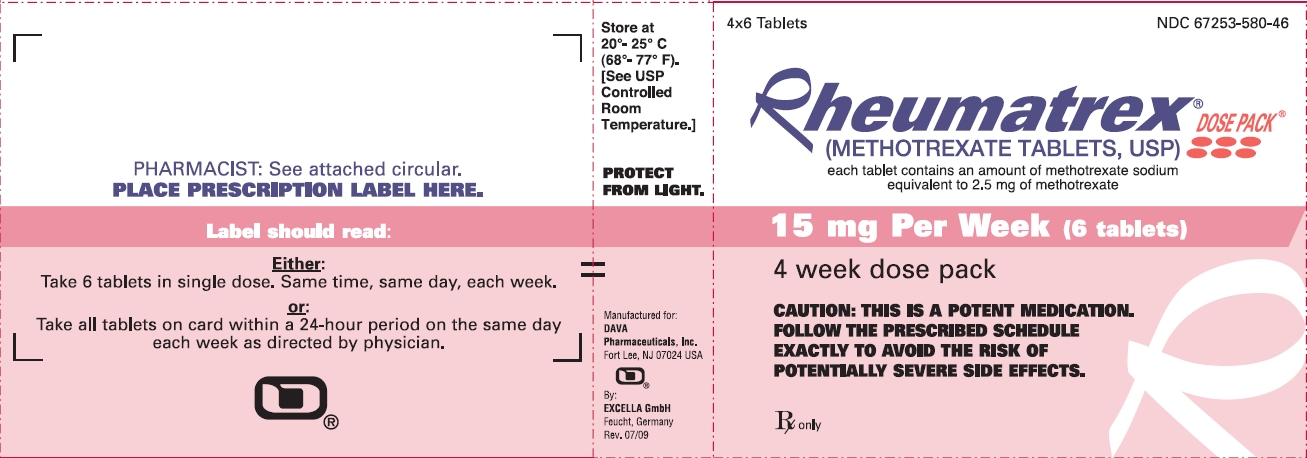
4x6 Tablets NDC 67253-580-46
Rheumatrex® DOSE PACK®
(METHOTREXATE TABLETS, USP)
each tablet contains an amount of methotrexate sodium equivalent to 2.5 mg of methotrexate
15 mg Per Week (6 tablets)
4 week dose pack
CAUTION: THIS IS A POTENT MEDICATION. FOLLOW THE PRESCRIBED SCHEDULE EXACTLY TO AVOID THE RISK OF POTENTIALLY SEVERE SIDE EFFECTS.
Rx only
Store at 20° - 25°C (68° - 77°F).
[See USP Controlled Room Temperature.]
PROTECT FROM LIGHT.
Manufactured for: DAVA Pharmaceuticals, Inc., Fort Lee, NJ 07024, USA
By: Excella GmbH, Feucht, Germany
Rev. 07/09
PHARMACIST: See attached circular.
PLACE PRESCRIPTION LABEL HERE.
Label should read:
Either:
Take 6 tablets in single dose. Same time, same day, each week.
or:
Take all tablets on card within a 24-hour period on the same day each week as directed by physician.