NDC Code(s) : 67091-200-24, 67091-200-10
Packager : WinCo Foods, LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Allergy Relief Diphenhydramine HCl TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - WinCo Foods, LLC(056098817) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 038154464 | pack(67091-200) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 832867837 | manufacture(67091-200), pack(67091-200) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 832867894 | manufacture(67091-200) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 868734088 | manufacture(67091-200) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 967626305 | pack(67091-200) | |
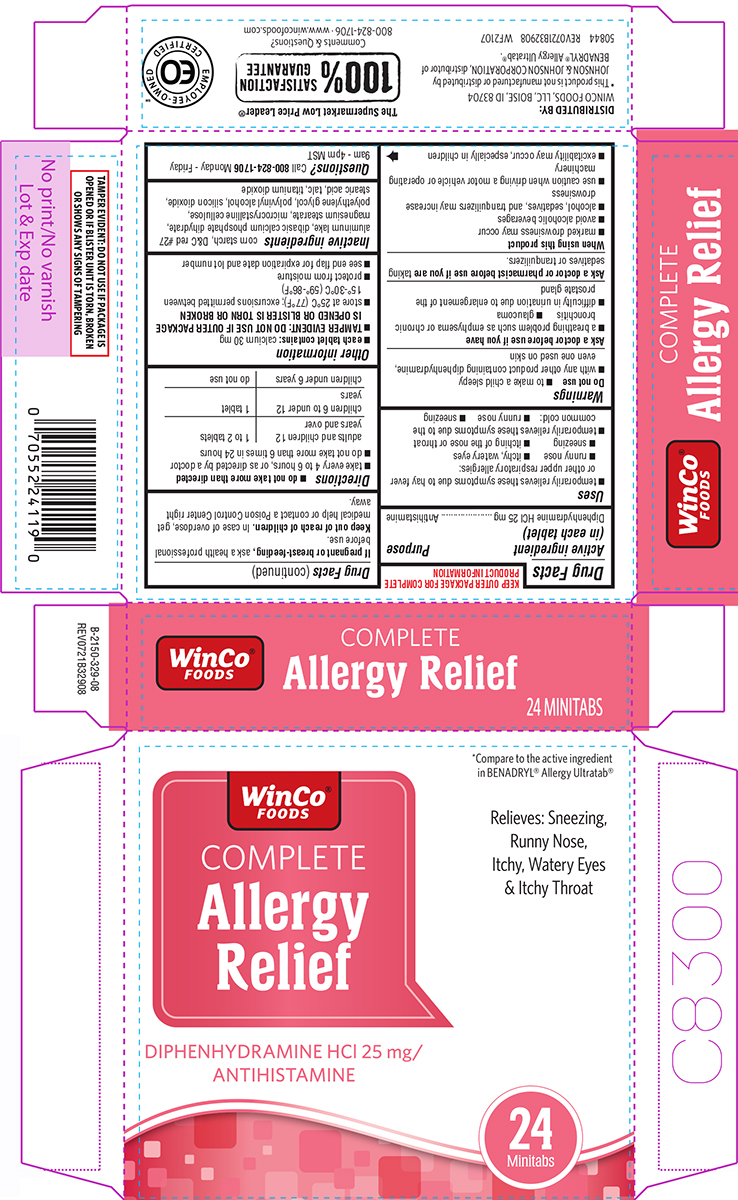
PRINCIPAL DISPLAY PANEL
WinCo®
FOODS
*Compare to the active ingredient
in BENADRYL® Allergy Ultratab®
COMPLETE
Allergy
Relief
DIPHENHYDRAMINE HCl 25 mg/
ANTIHISTAMINE
Relieves: Sneezing,
Runny Nose,
Itchy, Watery Eyes
& Itchy Throat
24
Minitabs
DISTRIBUTED BY:
WINCO FOODS, LLC, BOISE, ID 83704
*This product is not manufactured or distributed by
JOHNSON & JOHNSON CORPORATION, distributor of
BENADRYL® Allergy Ultratab®.
50844 REV0721B32908 WF2107
The Supermarket Low Price Leader®
100% SATISFACTION GUARANTEE
Comments & Questions? 800-824-1706
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN, BROKEN
OR SHOWS ANY SIGNS OF TAMPERING