NDC Code(s) : 66975-600-16, 66975-600-04
Packager : Benco Dental
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Chlorhexidine GluconateChlorhexidine Gluconate RINSE | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Benco Dental(015108087) |
| REGISTRANT - Xttrium Laboratories, Inc.(007470579) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Xttrium Laboratories, Inc. | 007470579 | manufacture(66975-600) | |
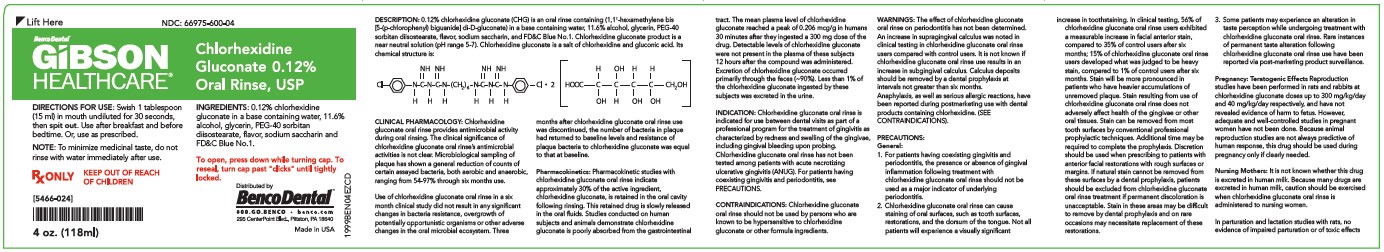
PRINCIPAL DISPLAY PANEL
NDC #66975-600-16
GIBSON™ Healthcare
Chlorhexidine Gluconate 0.12% Oral Rinse, USP, Mint
DIRECTIONS FOR USE: Fill cap to the "fill line" (15 ml). Swish in mouth undiluted for 30 seconds, then spit out. Use after breakfast and before bedtime. Or, use as prescribed. NOTE: Do not rinse with wate rimmediately after use.
1 Pint (473 mL)
KEEP OUT OF REACH OF CHILDREN
Rx
INGREDIENTS: 0.12% chlorhexidine gluconate in a base containing water, 11.6% alcohol, glycerin, PEG-40 diisostearate, flavor, sodium saccharin and FD&C Blue No.1.
To open, press in flat panels while turning cap.
To reseal, turn cap past "clicks" until tightly locked.
WHAT TO EXPECT WHEN USING CHLORHEXIDINE GLUCONATE ORAL RINSE
Your dentist has prescribed chlorhexidine gluconate oral rinse to treat your gingivitis, to help reduce the redness and swelling of your gums, and also to help you control any gum bleeding. Use chlorhexidine gluconate oral rinse regularly, as directed by your dentis, in addition to daily brushing. Spit out after use, chlorhexidine gluconate oral rinse should not be swallowed.
If you develop allergic symptoms such as skin rash, itch, generalized swelling, breathing difficulties, light headedness, rapid heart rate, upset stomach or diarrhea, seek medical attention immediately. Chlorhexidine gluconate oral rinse should not be used by persons who have a sensitivity to it or its components.
Chlorhexidine gluconate oral rinse may cause some tooth discoloration, or increase in tartar (calculus) formation, particularly in areas where stain and tartar usually form. it is important to see your dentist for removal of any stain or tartar at least every six months or more frequently if your dentist advises
- Both stain and tartar can be removed by your dentist or hygienist. Chlorhexidine gluconate oral rinse may cause permanent discoloration of some front-tooth fillings.
- To minimize discoloration, you should brush and floss daily, emphasinzing areas which begin to discolor.
- Chlorhexidine gluconate oral rinse may taste bitter to some patients and can affect how foods and beverages taste. This will become less noticeable in most cases with continued use of chlorhexidine gluconate oral rinse.
- To avoid taste interference, rinse with chlorhexidine gluconate oral rinse after meals. Do not rinse with water or other mouthwashes immediately after rinsing with chlorhexidine gluconate oral rinse.
If you have any questions or comments about chlorhexidine gluconate oral rinse, contact your dentist, pharmacist or Xtrium Laboratories, Inc. toll free at 1-800-587-3721. Call your health care provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Store at 20°C to 25°C (68°F to 77°F), excursions permitted toi 15°C to 30 °C (59°F to 86°F) [See USP controlled Room Temperature].
Distributed by Benco Dental
"We deliver success smile after smile"
800.GO.BENCO
Benco.com
295 CenterPoint Blvd., Pittston, PA 18640
.
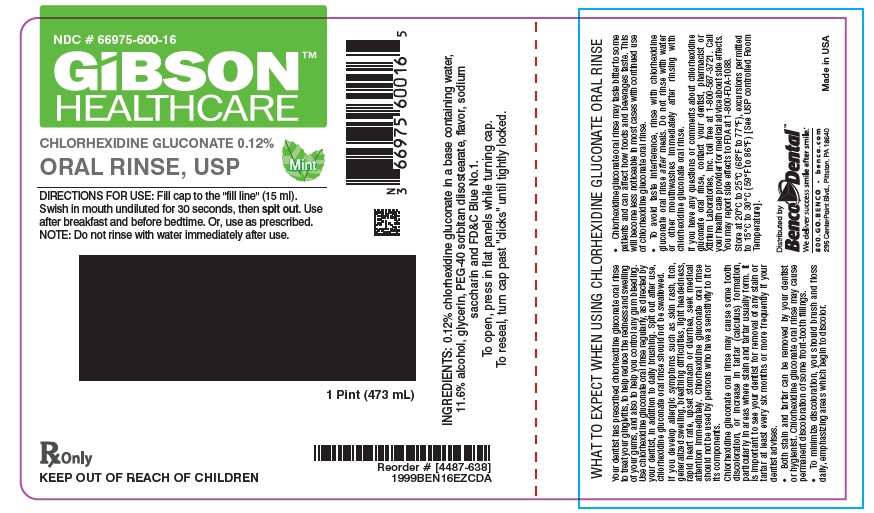


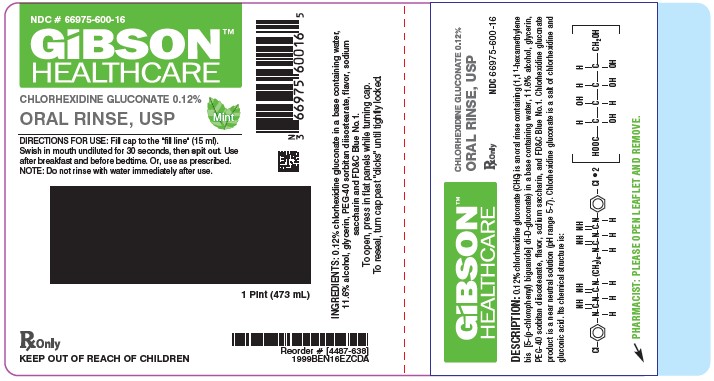
PRINCIPAL DISPLAY PANEL
NDC: 66975-600-04
BencoDental
GIBSON HEALTHCARE
Chlorhexidine Gluconate 0.12% Oral Rinse, USP
DIRECTIONS FOR USE: Swish 1 tablespoon (15 ml) in mouth undiluted for 30 seconds, then spit out. Use after breakfast and before bedtime. Or, use as prescribed.
NOTE: To minimize medicinal taste, do not rinse with water immediately after use.
INGREDIENTS: 0.12% chlorhexidine gluconate in a base containing water, 11.6% alcohol, glycerin, PEG-40 sorbitan diisostearate, flavor, sodium saccharin and FD&C Blue No. 1.
RX ONLY
KEEP OUT OF REACH OF CHILDREN
To open, pressdown while turning cap. to reseal, turn cap past "clicks" until tightly locked.
[5466-024]
4 oz. (118ml)
Distributed by
BencoDental
800.GO.BENCO benco.com
295 CenterPoint Blvd., Pittston, PA 18640
Made in USA
1999BEN04EZCD