NDC Code(s) : 64950-362-04
Packager : Genus Lifesciences Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| cocaine hydrochloridecocaine hydrochloride SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Genus Lifesciences Inc.(113290444) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Genus Lifesciences Inc. | 113290444 | manufacture(64950-362) | |
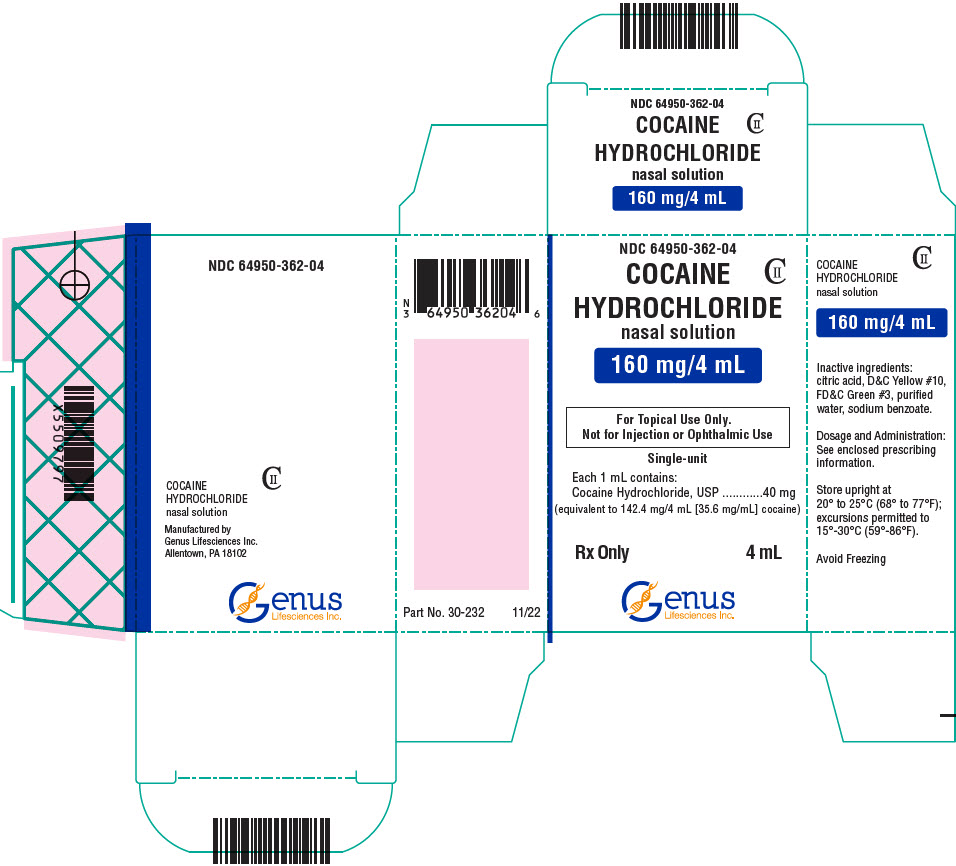
PRINCIPAL DISPLAY PANEL
NDC 64950-362-04
COCAINE
HYDROCHLORIDE
nasal solution
CII
160 mg/4 mL
For Topical Use Only.
Not for Injection or Ophthalmic Use
Single-unit
Each 1 mL contains:
Cocaine Hydrochloride, USP
40 mg
(equivalent to 142.4 mg/4 mL [35.6 mg/mL] cocaine)
Rx Only
4 mL
Genus
Lifesciences Inc.