NDC Code(s) : 63981-329-78
Packager : COSTCO WHOLESALE CORPORATION
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Allergy MedicineDiphenhydramine HCl TABLET, FILM COATED | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LABELER - COSTCO WHOLESALE CORPORATION(103391843) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 038154464 | pack(63981-329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 832867837 | manufacture(63981-329), pack(63981-329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 832867894 | manufacture(63981-329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 868734088 | manufacture(63981-329) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 967626305 | pack(63981-329) | |
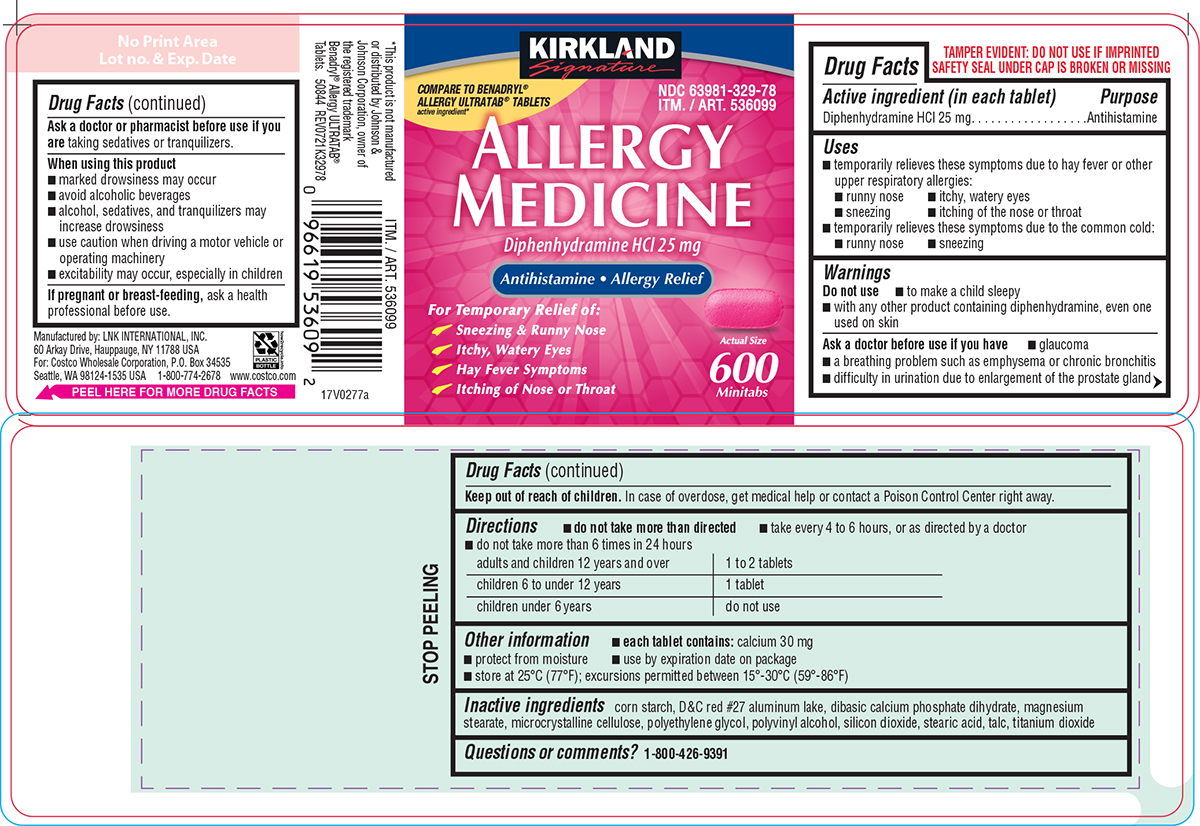
PRINCIPAL DISPLAY PANEL
KIRKLAND
Signature
COMPARE TO BENADRYL®
ALLERGY ULTRATAB® TABLETS
active ingredient*
NDC 63981-329-78
ITM. / ART. 536099
ALLERGY
MEDICINE
Diphenhydramine HCl 25 mg
Antihistamine • Allergy Relief
For Temporary Relief of:
Sneezing & Runny Nose
Itchy, Watery Eyes
Hay Fever Symptoms
Itching of Nose or Throat
Actual Size
600
Minitabs
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured
or distributed by Johnson &
Johnson Corporation, owner of
the registered trademark
Benadryl® Allergy ULTRATAB®
Tablets. 50844 REV0721K32978
Manufactured by: LNK INTERNATIONAL, INC.
60 Arkay Drive, Hauppauge, NY 11788 USA
For: Costco Wholesale Corporation, P.O. Box 34535
Seattle, WA 98124-1535 USA 1-800-774-2678 www.costco.com
 Kirkland 44-329
Kirkland 44-329









