NDC Code(s) : 63459-910-01, 63459-910-12, 63459-910-11, 63459-910-15, 63459-910-18, 63459-910-17, 63459-910-36, 63459-912-01, 63459-912-12, 63459-912-11, 63459-912-15, 63459-912-18, 63459-912-17, 63459-912-36, 63459-918-53, 63459-918-59, 63459-920-53, 63459-920-59
Packager : Cephalon, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| GRANIXtbo-filgrastim INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRANIXtbo-filgrastim INJECTION, SOLUTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| GRANIXtbo-filgrastim INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| GRANIXtbo-filgrastim INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Cephalon, LLC(183236314) |
PRINCIPAL DISPLAY PANEL
NDC 63459-910-11
Rx only
Granix® (tbo-filgrastim) Injection
300 mcg/0.5 mL
For Subcutaneous Use Only
A recombinant Granulocyte Colony-Stimulating Factor (rG-CSF) derived from E Coli
300 mcg/0.5 mL
For Healthcare Professional Use Only
1 Single-dose prefilled syringe with a safety needle guard
Discard unused portion

PRINCIPAL DISPLAY PANEL
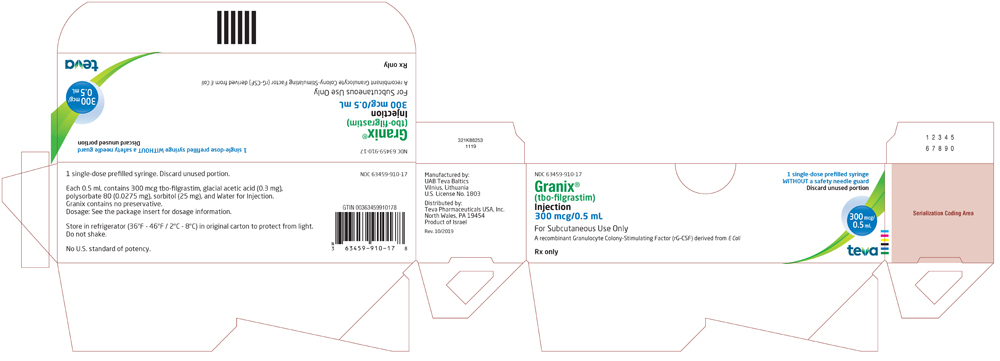
NDC 63459-910-17
Granix® (tbo-filgrastim) Injection
300 mcg/0.5 mL
For Subcutaneous Use Only
A recombinant Granulocyte Colony-Stimulating Factor (rG-CSF) derived from E Coli
1 Single-dose prefilled syringe WITHOUT a safety needle guard
Rx only
Discard unused portion
300 mcg/0.5 mL
PRINCIPAL DISPLAY PANEL
NDC 63459-912-11
Rx only
Granix® (tbo-filgrastim) Injection
480 mcg/0.8 mL
For Subcutaneous Use Only
A recombinant Granulocyte Colony-Stimulating Factor (rG-CSF) derived from E Coli
480 mcg/0.8 mL
For Healthcare Professional Use Only
1 Single-dose prefilled syringe with a safety needle guard
Discard unused portion

PRINCIPAL DISPLAY PANEL
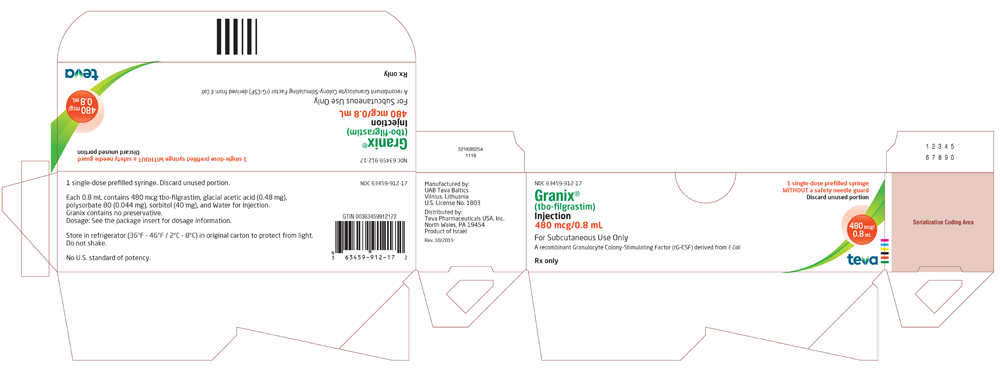
NDC 63459-912-17
Rx only
Granix® (tbo-filgrastim) Injection
480 mcg/0.8 mL
For Subcutaneous Use Only
A recombinant Granulocyte Colony-Stimulating Factor (rG-CSF) derived from E Coli
480 mcg/0.8 mL
For Healthcare Professional Use Only
1 Single-dose prefilled syringe WITHOUT a safety needle guard
Discard unused portion
PRINCIPAL DISPLAY PANEL
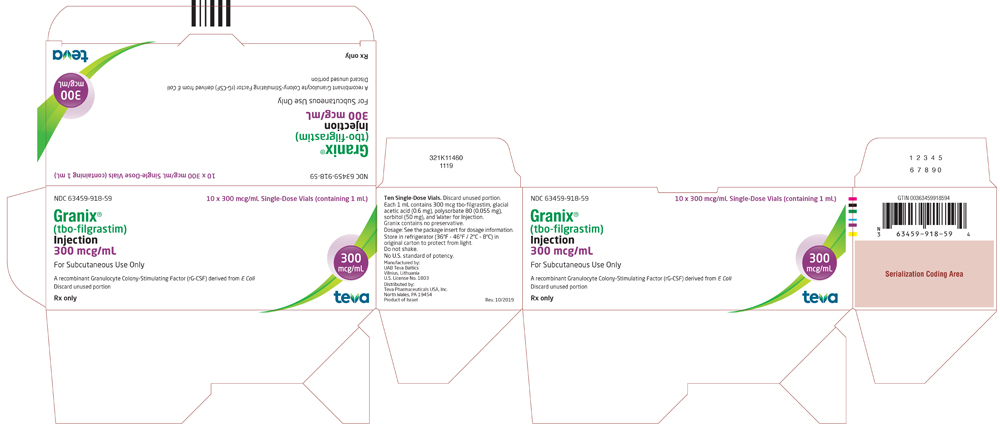
NDC 63459-918-59
10 x 300 mcg/mL Single-Dose Vials (containing 1 mL)
Granix (tbo-filgrastim) Injection
300 mcg/mL
For Subcutaneous Use Only.
A recombinant Granulocyte Colony-Stimulating Factor (rG-CSF) derived from E. coli. Discard unused portion.
Rx Only
PRINCIPAL DISPLAY PANEL
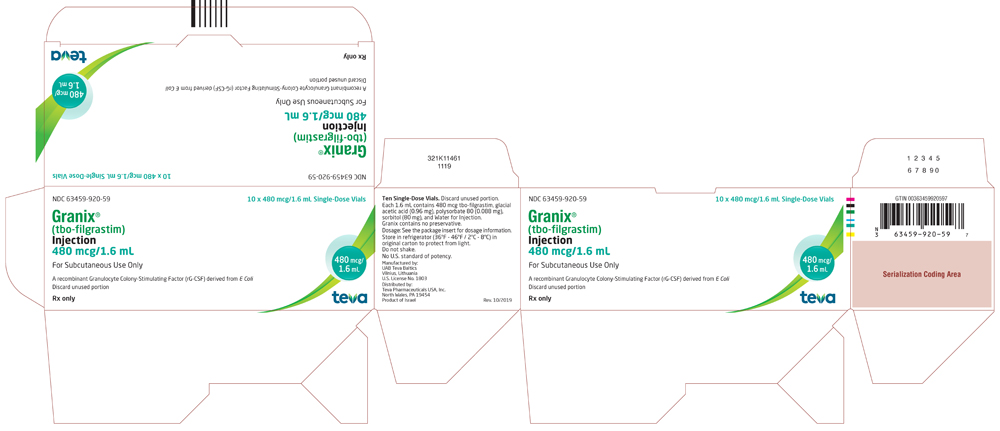
NDC 63459-920-59
10 x 480 mcg/1.6 mL Single-Dose Vials
Granix (tbo-filgrastim) Injection
480 mcg/1.6 mL
For Subcutaneous Use Only. A recombinant Granulocyte Colony-Stimulating Factor (rG-CSF) derived from E. coli. Discard unused portion.
Rx Only