NDC Code(s) : 63323-270-26
Packager : Fresenius Kabi USA, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| PropofolPROPOFOL INJECTION, EMULSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
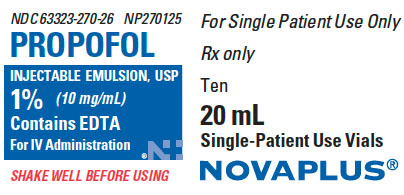
PRINCIPAL DISPLAY PANEL
P ACKAGE LABEL - PRINCIPAL DISPLAY - Propofol 20 mL Vial Label
NDC 63323-270-26
NP270125
PROPOFOL INJECTABLE EMULSION, USP
1% (10 mg/mL)
Contains EDTA
For IV Administration
SHAKE WELL BEFORE USING
20 mL Vial
Rx Only

P ACKAGE LABEL - PRINCIPAL DISPLAY - Propofol 20 mL Vial Carton Label
NDC 63323-270-26
NP270125
PROPOFOL INJECTABLE EMULSION, USP
1% (10 mg/mL)
Contains EDTA
For IV Administration
SHAKE WELL BEFORE USING
For Single Patient Use Only
Rx only
Ten 20 mL Single-Patient Use Vials