NDC Code(s) : 63323-172-60
Packager : Fresenius Kabi USA, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Carboplatin CARBOPLATIN INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Fresenius Kabi USA, LLC(608775388) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Fresenius Kabi USA, LLC | 023648251 | analysis(63323-172), manufacture(63323-172) | |
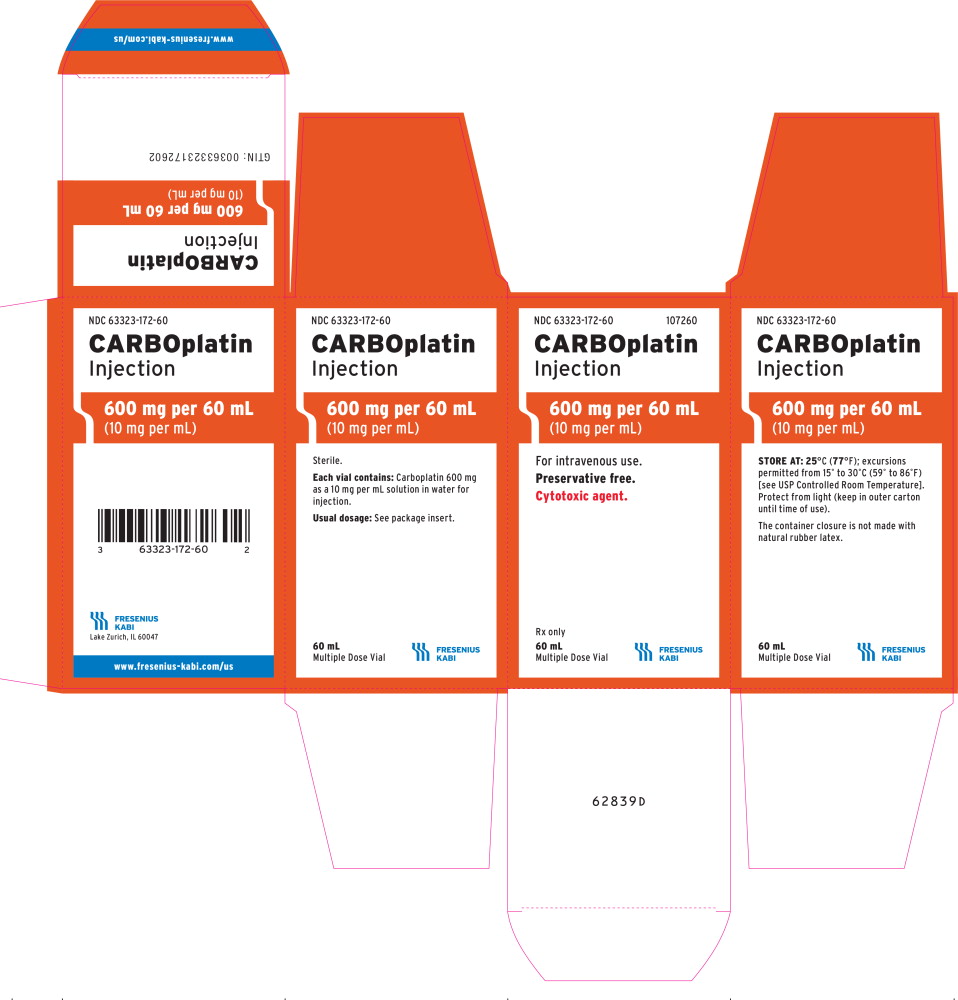
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL - PRINCIPAL DISPLAY - Carboplatin 600 mg per 60 mL Multiple Dose Vial Label
NDC 63323-172-60 107260
CARBOplatin Injection
600 mg per 60 mL
(10 mg per mL)
For intravenous use.
Preservative free.
Cytotoxic agent.
60 mL
Multiple Dose Vial Rx only
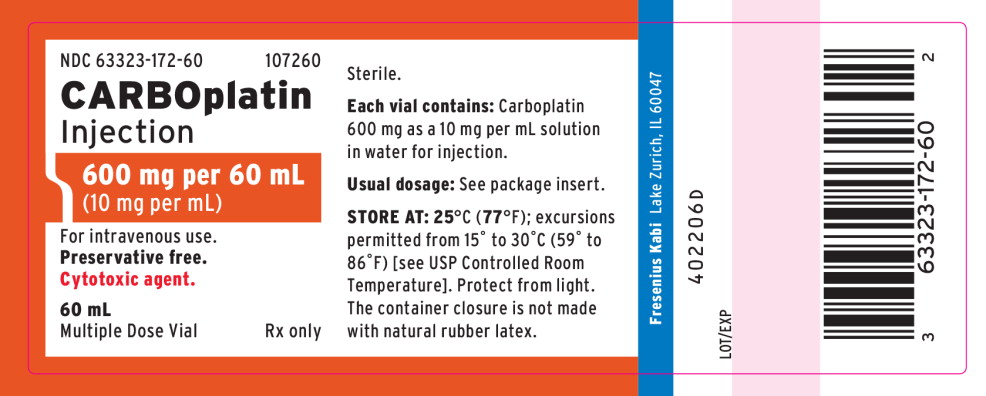
PACKAGE LABEL - PRINCIPAL DISPLAY - Carboplatin 600 mg per 60 mL Multiple Dose Vial Carton Panel
NDC 63323-172-60 107260
CARBOplatin Injection
600 mg per 60 mL
(10 mg per mL)
For intravenous use.
Preservative free.
Cytotoxic agent.
Rx only
60 mL
Multiple Dose Vial