NDC Code(s) : 63304-723-30, 63304-723-90, 63304-723-05
Packager : Sun Pharmaceutical Industries, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Bupropion hydrochlorideBupropion hydrochloride TABLET, EXTENDED RELEASE | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| LABELER - Sun Pharmaceutical Industries, Inc.(146974886) |
| REGISTRANT - Sun Pharmaceutical Industries, Inc.(146974886) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Sun Pharmaceutical Industries Limited | 650456002 | MANUFACTURE(63304-723) | |
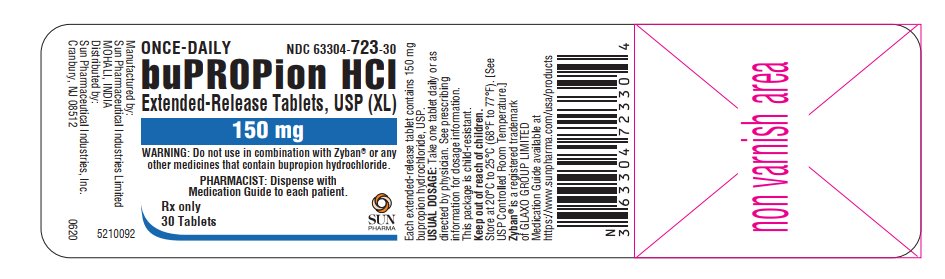
PRINCIPAL DISPLAY PANEL
ONCE-DAILY
NDC 63304-723-30
buPROPion HCl Extended-Release Tablets, USP (XL)
150 mg
WARNING: Do not use in combination with Zyban® or any other medicines that contain bupropion hydrochloride
PHARMACIST: Dispense with Medication Guide to each patient
Rx only
- 30 tablets SUN PHARMA
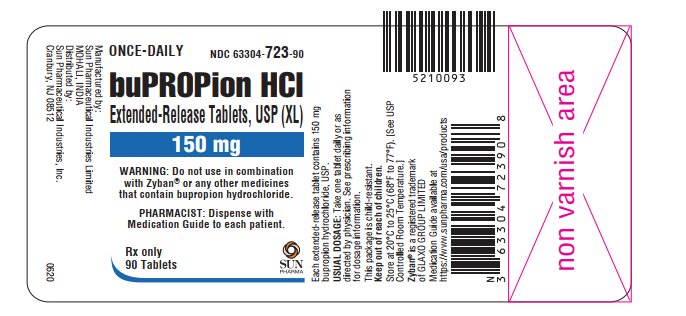
PRINCIPAL DISPLAY PANEL
ONCE-DAILY
NDC 63304-723-90
buPROPion HCl Extended-Release Tablets, USP (XL)
150 mg
WARNING: Do not use in combination with Zyban® or any other medicines that contain bupropion hydrochloride
PHARMACIST: Dispense with Medication Guide to each patient
Rx only
- 90 tablets SUN PHARMA
PRINCIPAL DISPLAY PANEL
ONCE-DAILY
NDC 63304-723-05
buPROPion HCl Extended-Release Tablets, USP (XL)
150 mg
WARNING: Do not use in combination with Zyban® or any other medicines that contain bupropion hydrochloride
PHARMACIST: Dispense with Medication Guide to each patient
Rx only
- 500 tablets SUN PHARMA










