NDC Code(s) : 62856-250-01, 62856-250-10, 62856-500-01, 62856-500-10, 62856-750-01, 62856-750-10, 62856-100-01, 62856-100-10, 62856-101-01, 62856-101-10, 62856-125-01, 62856-125-10, 62856-150-01, 62856-150-10, 62856-180-01, 62856-180-10, 62856-251-01, 62856-102-01
Packager : Eisai Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Fragmindalteparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Fragmindalteparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Fragmindalteparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Fragmindalteparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Fragmindalteparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Fragmindalteparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Fragmindalteparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Fragmindalteparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Fragmindalteparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Fragmindalteparin sodium INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 62856-250-10 (Single-dose prefilled syringes)

PRINCIPAL DISPLAY PANEL
NDC 62856-500-10 (Single-dose prefilled syringes)

PRINCIPAL DISPLAY PANEL
NDC 62856-750-10 (Single-dose prefilled syringes)

PRINCIPAL DISPLAY PANEL
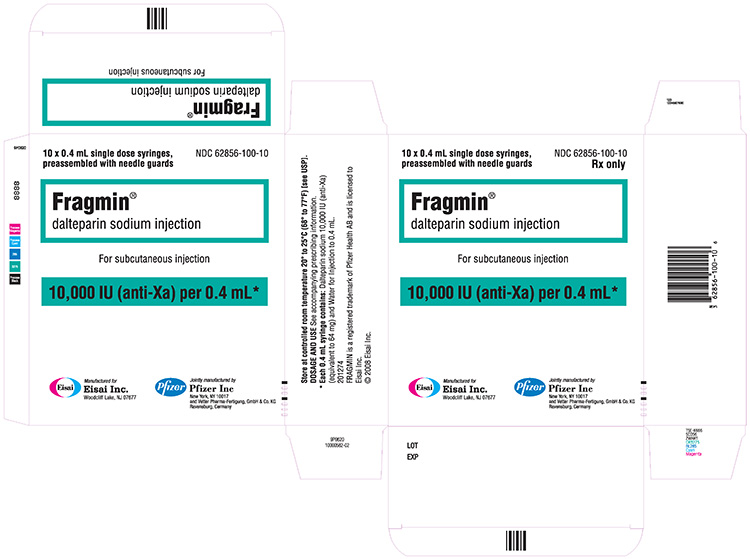
NDC 62856-100-10 (Single-dose prefilled syringes)
PRINCIPAL DISPLAY PANEL
NDC 62856-101-10 (Single-dose graduated syringes)

PRINCIPAL DISPLAY PANEL
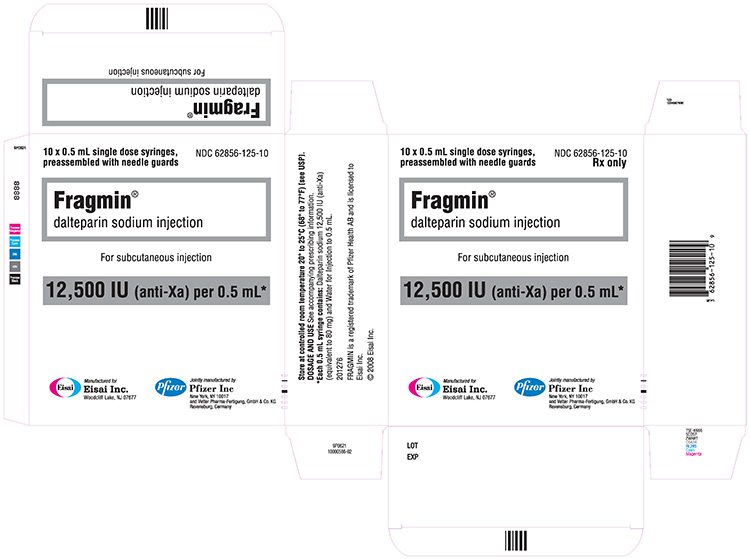
NDC 62856-125-10 (Single-dose prefilled syringes)
PRINCIPAL DISPLAY PANEL
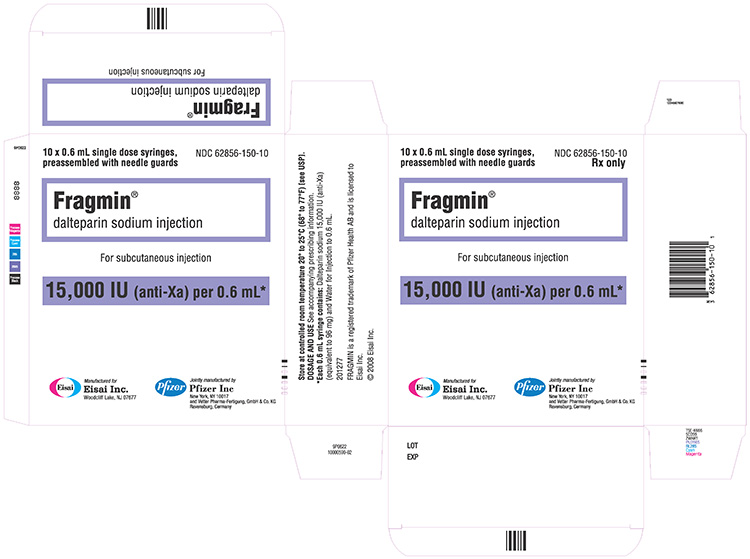
NDC 62856-150-10 (Single-dose prefilled syringes)
PRINCIPAL DISPLAY PANEL
NDC 62856-180-10 (Single-dose prefilled syringes)

PRINCIPAL DISPLAY PANEL
NDC 62856-251-01 (Multiple dose vial)
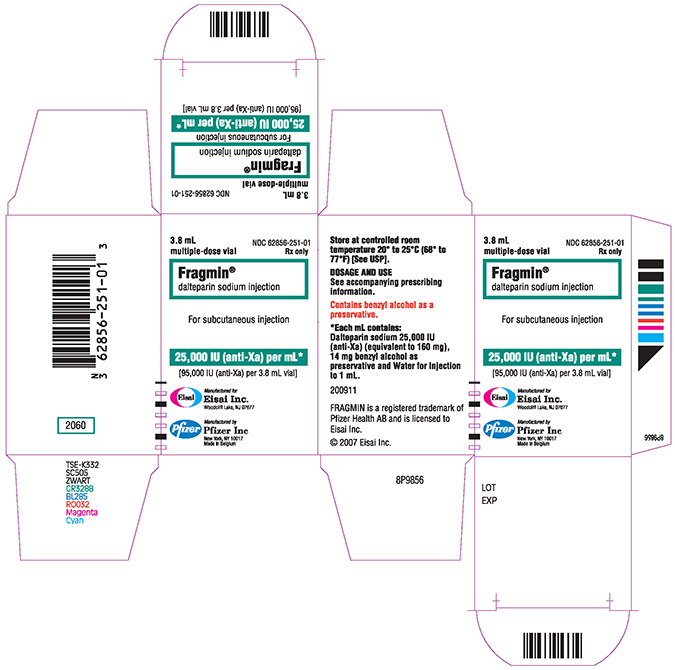
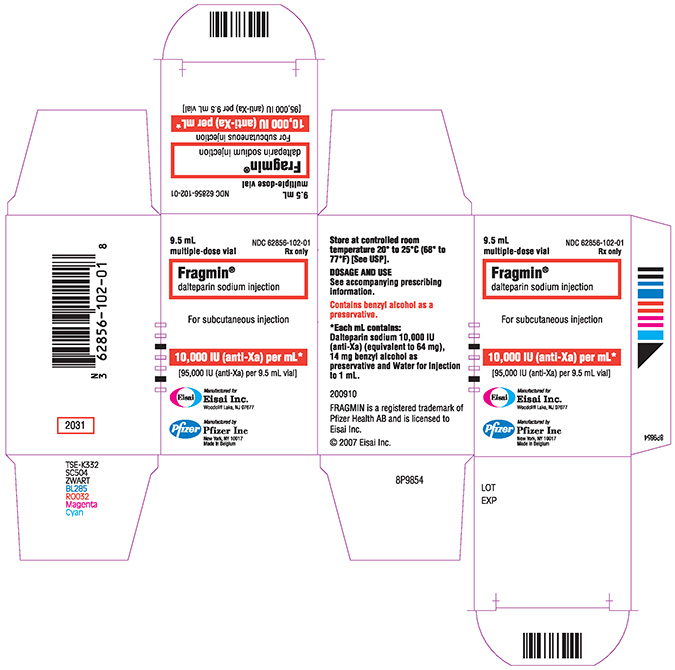
PRINCIPAL DISPLAY PANEL
NDC 62856-102-01 (Multiple dose vial)