NDC Code(s) : 62856-245-30, 62856-245-90, 62856-245-41, 62856-245-11, 62856-246-11, 62856-246-30, 62856-246-90, 62856-246-41, 62856-247-30, 62856-831-30, 62856-832-30
Packager : Eisai Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Ariceptdonepezil hydrochloride TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Ariceptdonepezil hydrochloride TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Ariceptdonepezil hydrochloride TABLET, FILM COATED | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Aricept donepezil hydrochloride TABLET, ORALLY DISINTEGRATING | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Aricept donepezil hydrochloride TABLET, ORALLY DISINTEGRATING | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Eisai Inc.(189246791) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Catalent Pharma Solutions, LLC | 014167995 | analysis(62856-245, 62856-246, 62856-247, 62856-831, 62856-832) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Eisai Co., Ltd. | 695153247 | analysis(62856-247), manufacture(62856-247) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Eisai Co., Ltd. | 695319983 | analysis(62856-245, 62856-246, 62856-247, 62856-831, 62856-832), api manufacture(62856-245, 62856-246, 62856-247, 62856-831, 62856-832) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Eisai Manufacturing Ltd | 219516916 | analysis(62856-245, 62856-246), manufacture(62856-245, 62856-246) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pharma Packaging Solutions, LLC dba Tjoapack, LLC | 928861723 | label(62856-245, 62856-246, 62856-247), pack(62856-245, 62856-246, 62856-247) | |
PRINCIPAL DISPLAY PANEL
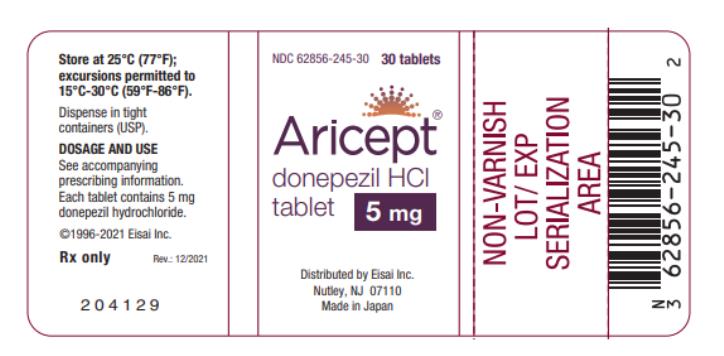
PRIN CIPAL DISPLAY PANEL - ARICEPT 5 MG TABLETS
NDC 62856-245-30
Aricept®
donepezil HCl tablet
5 mg
30 Tablets
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - ARICEPT 10 MG TABLETS
NDC 62856-246-30
Aricept®
donepezil HCl tablet
10 mg
30 Tablets

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - ARICEPT 23 MG TABLETS
NDC 62856-247-30
Aricept®
donepezil HCl tablet
23 mg
30 tablets

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - ARICEPT ODT 5 MG TABLETS
NDC 62856-831-30
ARICEPT® ODT™
(donepezil HCl) 5 MG
orally disintegrating tablets

PRINCIPAL DISPLAY PANEL
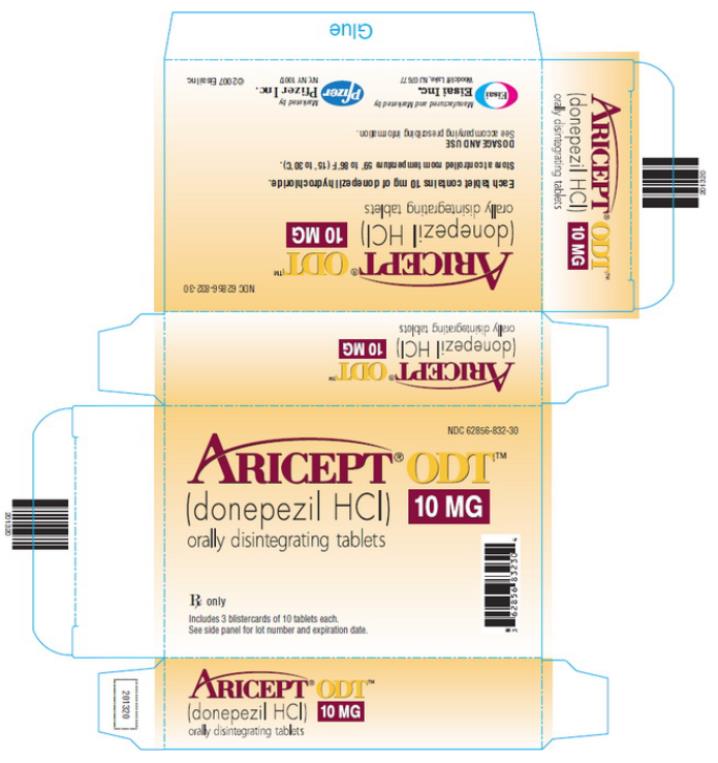
PRINCIPAL DISPLAY PANEL - ARICEPT ODT 10 MG TABLETS
NDC 62856-832-30
ARICEPT® ODT™
(donepezil HCl) 10 MG
orally disintegrating tablets