NDC Code(s) : 62332-251-30, 62332-251-06, 62332-251-18
Packager : Alembic Pharmaceuticals Inc.
Category : Human Prescription Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Azithromycin Azithromycin TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| LABELER - Alembic Pharmaceuticals Inc.(079288842) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Alembic Pharmaceuticals Limited | 650574671 | MANUFACTURE(62332-251) | |
PRINCIPAL DISPLAY PANEL
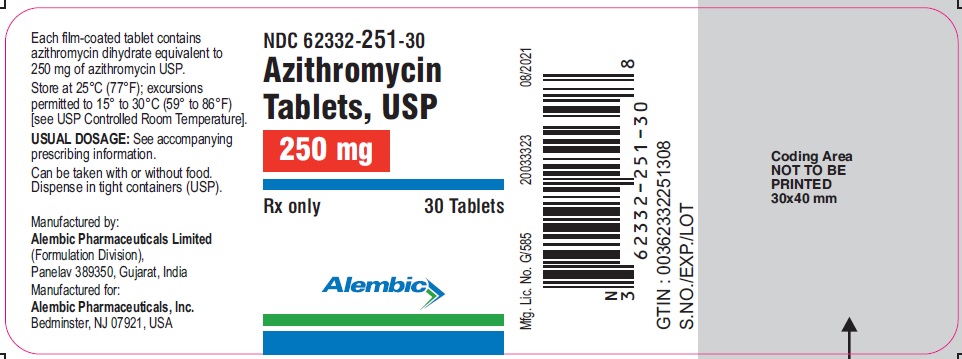
NDC 62332-251-30
Azithromycin
Tablets, USP
250 mg
Rx only
30 Tablets
Alembic
PRINCIPAL DISPLAY PANEL
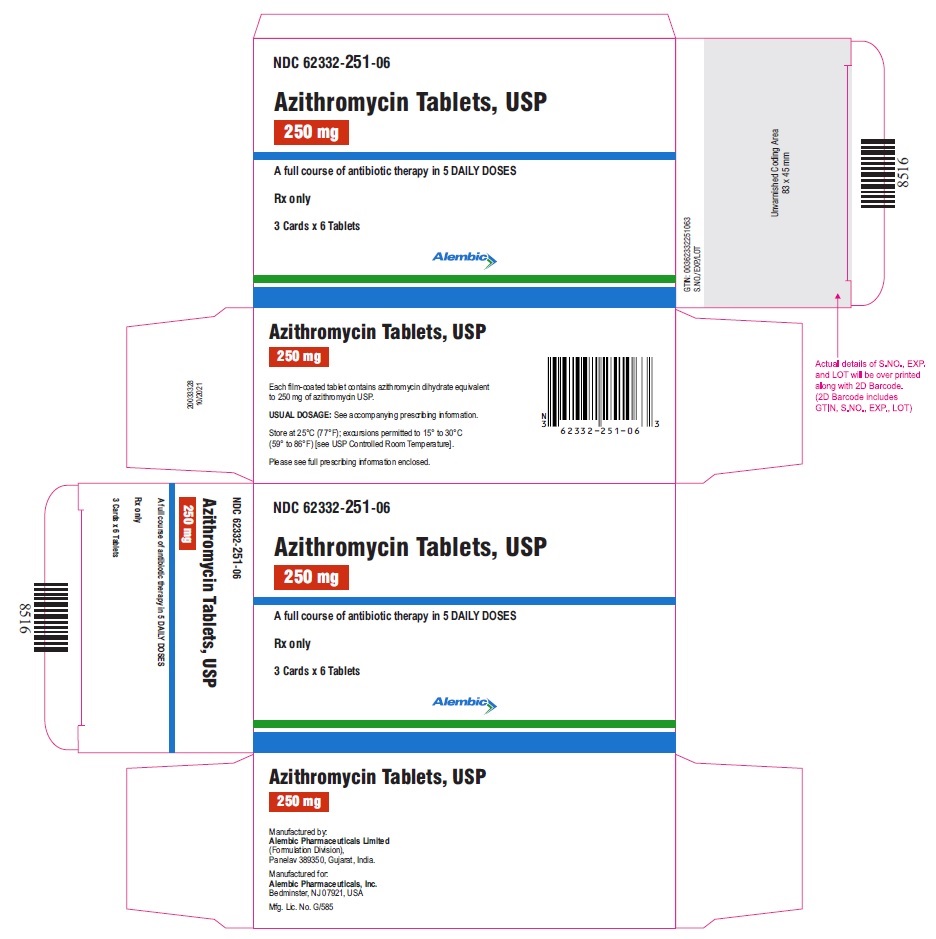
NDC 62332-251-06
Azithromycin Tablets, USP
250 mg
A full course of antibiotic therapy in 5 DAILY DOSES
Rx only
1 Card x 6 Tablets
Alembic

PRINCIPAL DISPLAY PANEL
NDC 62332-251-06
Azithromycin Tablets, USP
250 mg
A full course of antibiotic therapy in 5 DAILY DOSES
Rx only
Alembic
3 Cards x 6 Tablets
PRINCIPAL DISPLAY PANEL
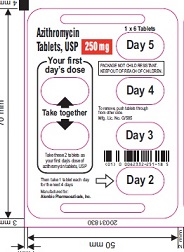
Azithromycin Tablets,
USP 250 mg
1 x 6 Tablets
PRINCIPAL DISPLAY PANEL
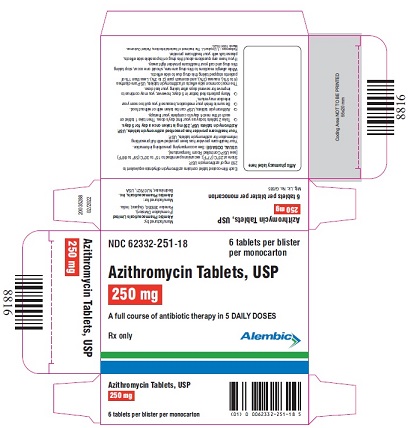
NDC 62332-251-18
6 tablets per blister
per monocarton
Azithromycin Tablets, USP
250 mg
A full course of antibiotic therapy in 5 DAILY DOSES
Rx only
Alembic
PRINCIPAL DISPLAY PANEL
Azithromycin Tablets, USP
250 mg
A full course of antibiotic therapy in 5 DAILY DOSES
3 monocartons (6 tablets per blister per monocarton)
Rx only
Alembic










