NDC Code(s) : 61874-002-26, 61874-002-72, 61874-002-30
Packager : Allergan, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| EMLAlidocaine and prilocaine CREAM | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
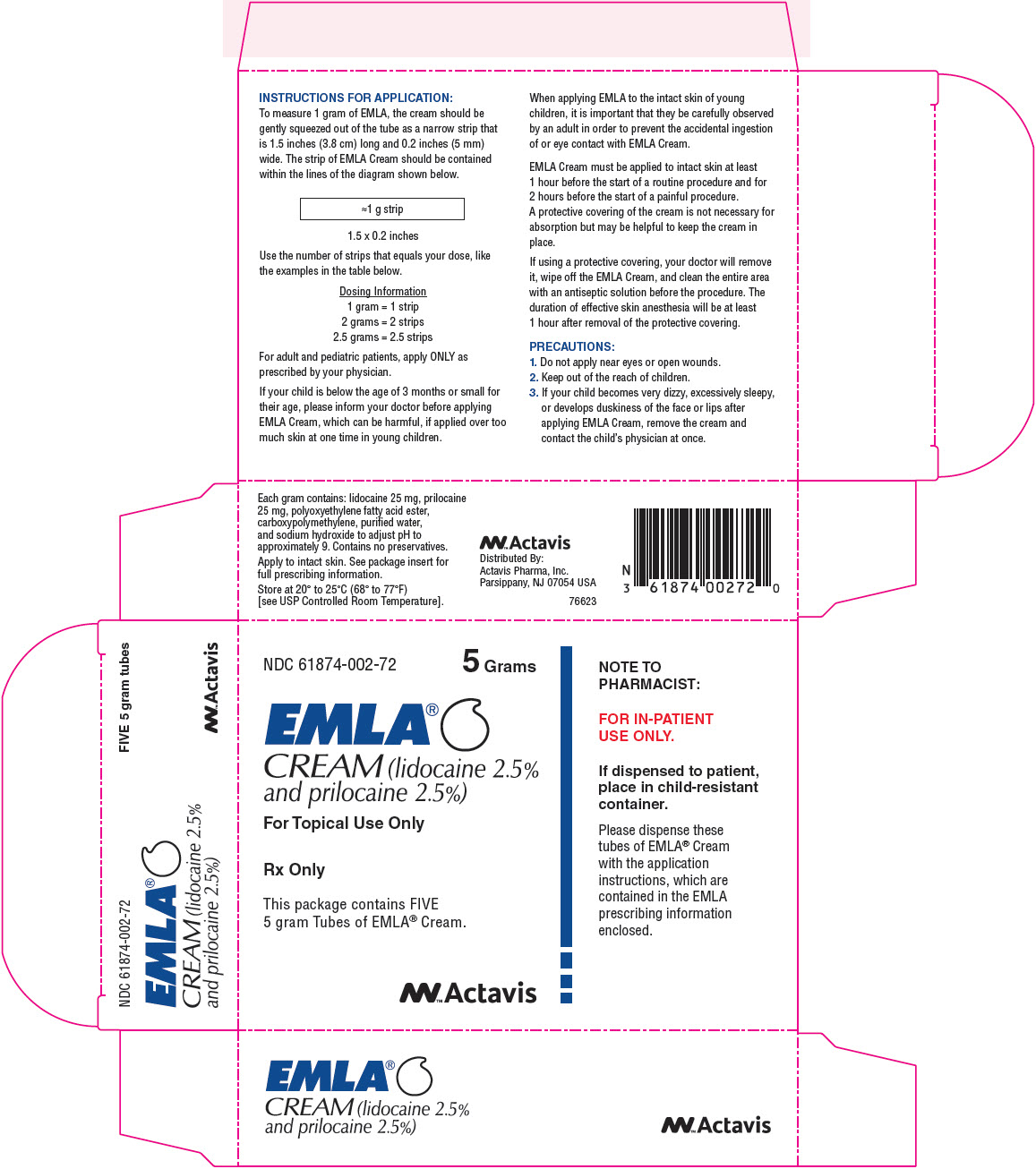
PRINCIPAL DISPLAY PANEL
NDC 61874-002-72 5 Grams
EMLA®
CREAM (lidocaine 2.5%
and prilocaine 2.5%)
For Topical Use Only
Rx only
PRINCIPAL DISPLAY PANEL
NDC 61874-002-30 30 Grams
EMLA®
CREAM (lidocaine 2.5%
and prilocaine 2.5%)
For Topical Use Only










