NDC Code(s) : 61570-037-75
Packager : Pfizer Laboratories Div Pfizer Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| VIROPTICtrifluridine SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
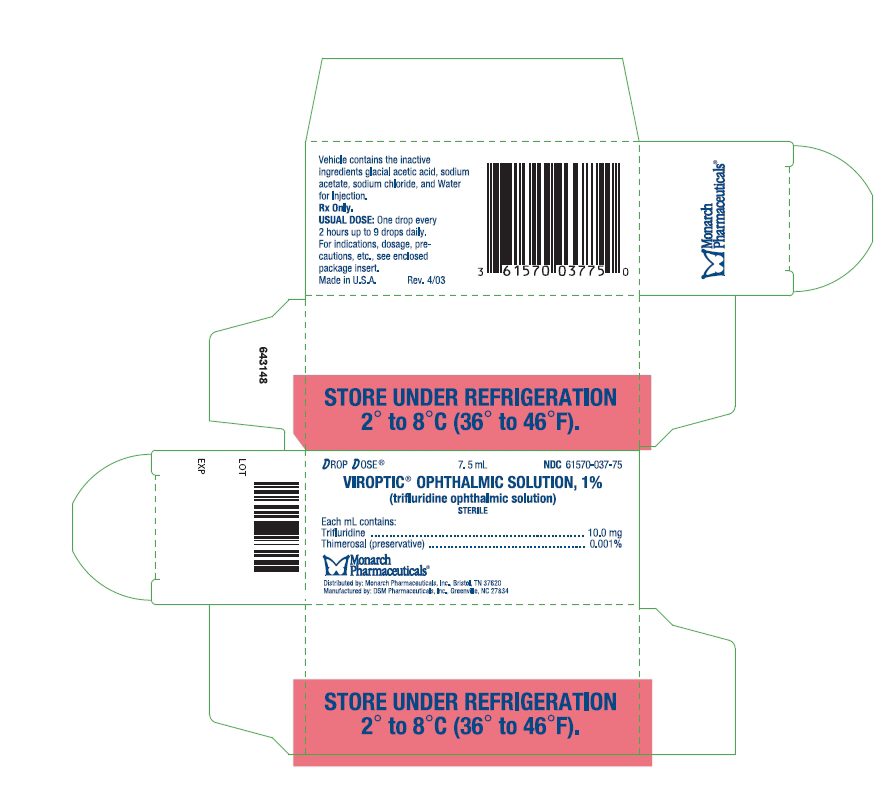
PRINCIPAL DISPLAY PANEL
Drop Dose®
7.5 mL
NDC 61570-037-75
VIROPTIC
® OPHTHALMIC SOLUTION, 1%
(trifluridine ophthalmic solution)
STERILE
Rx only.
STORE UNDER REFRIGERATION 2° to 8°C (36° to 46°F).
Monarch
Pharmaceuticals
®
Distributed by: Monarch
Pharmaceuticals, Inc., Bristol, TN 37620
Manufactured by: DSM Pharmaceuticals, Inc.,
Greenville, NC 27834
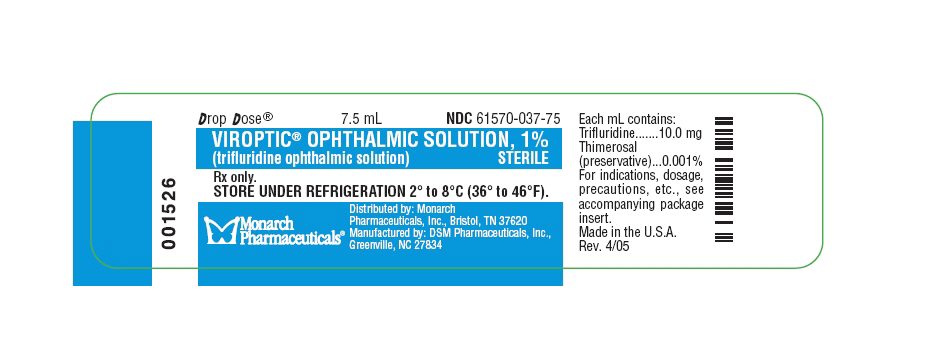
PRINCIPAL DISPLAY PANEL
DROP DOSE®
7.5 mL
NDC 61570-037-75
VIROPTIC
® OPHTHALMIC SOLUTION, 1%
(trifluridine ophthalmic solution)
STERILE
Each mL contains:
Trifluridine 10.0 mg
Thimerosal (preservative) 0.001%
Monarch
Pharmaceuticals
®
Distributed by: Monarch Pharmaceuticals, Inc., Bristol, TN 37620
Manufactured by: DSM Pharmaceuticals, Inc., Greenville, NC 27834