NDC Code(s) : 59779-923-03, 59779-923-08, 59779-923-22, 59779-923-12, 59779-923-51, 59779-923-07, 59779-923-06
Packager : CVS Pharmacy
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Allergy ReliefDiphenhydramine HCl TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - CVS Pharmacy(062312574) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 038154464 | pack(59779-923) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 832867837 | manufacture(59779-923), pack(59779-923) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 832867894 | manufacture(59779-923) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 868734088 | manufacture(59779-923) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 967626305 | pack(59779-923) | |
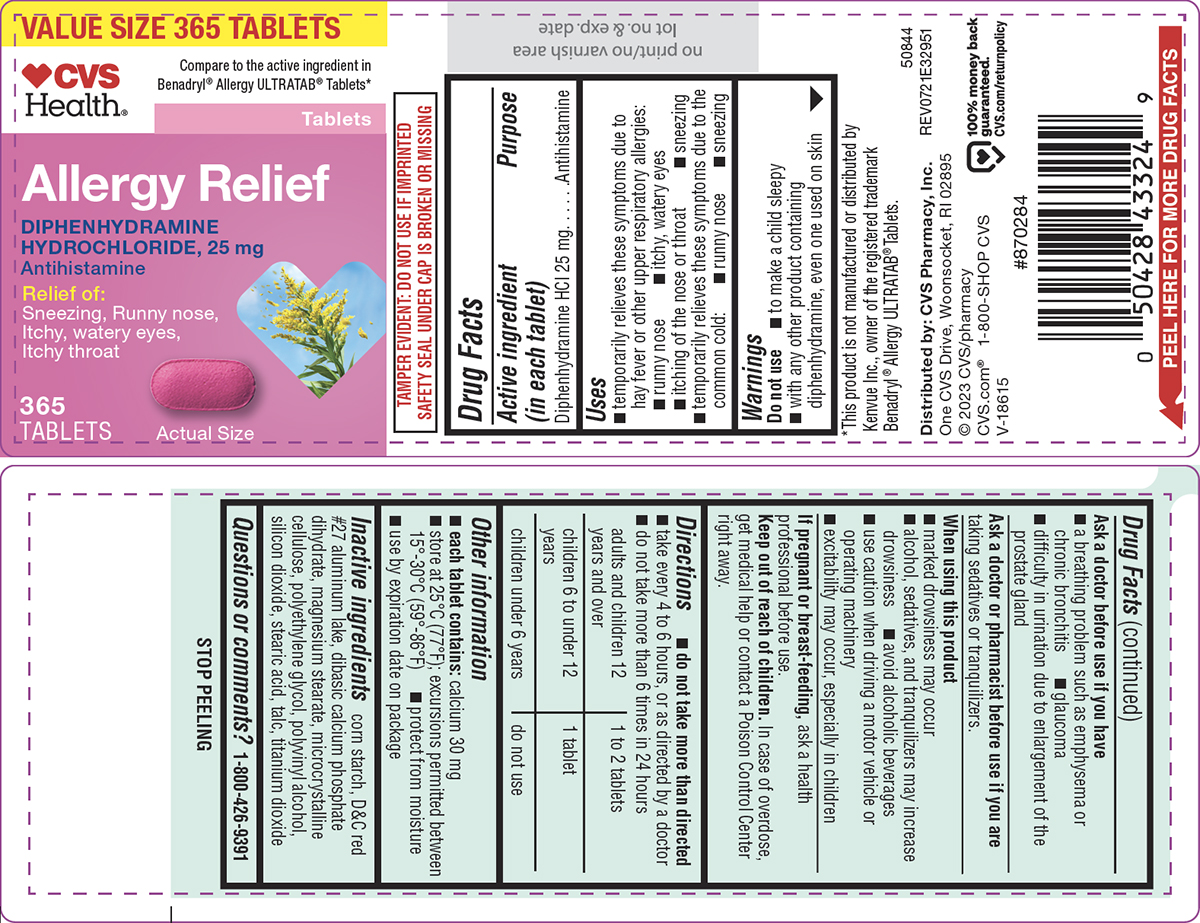
PRINCIPAL DISPLAY PANEL
VALUE SIZE 365 TABLETS
♥︎CVS
Health®
Compare to the active ingredient in
Benadryl® Allergy ULTRATAB® Tablets*
Allergy Relief
DIPHENHYDRAMINE
HYDROCHLORIDE, 25 mg
Antihistamine
Relief of:
Sneezing, Runny nose,
Itchy, watery eyes,
Itchy throat
365
TABLETS
Actual Size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by
Kenvue Inc., owner of the registered trademark
Benadryl® Allergy ULTRATAB® Tablets.
50844
REV0721E32951
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2023 CVS/pharmacy
CVS.com® 1-800-SHOP CVS
V-18615
100% money back
guaranteed.
CVS.com/return policy
 CVS 44-329
CVS 44-329