NDC Code(s) : 58160-976-02, 58160-976-20
Packager : GlaxoSmithKline Biologicals SA
Category : VACCINE LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BexseroNEISSERIA MENINGITIDIS SEROGROUP B NHBA FUSION PROTEIN ANTIGEN, NEISSERIA MENINGITIDIS SEROGROUP B FHBP FUSION PROTEIN ANTIGEN and NEISSERIA MENINGITIDIS SEROGROUP B NADA PROTEIN ANTIGEN INJECTION, SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - GlaxoSmithKline Biologicals SA(372748392) |
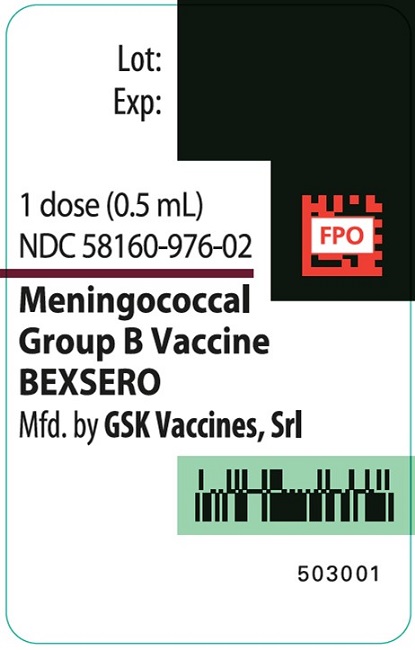
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 58160-976-20
BEXSERO
Meningococcal Group B Vaccine
MenB
GSK
Rx Only
For Use from 10 through 25 Years of Age
10 Disposable Single-Dose Prefilled TIP-LOK Syringes each containing one 0.5-mL dose
TIP-LOK syringes to be used with Luer Lock compatible needles
NEEDLES NOT INCLUDED
BEXSERO
Made in Italy
©2023 GSK group of companies or its licensor.
- Rev. 08/23
- 515763
-

PRINCIPAL DISPLAY PANEL
Syringe Label
NDC 58160-976-02
503001