NDC Code(s) : 56062-308-62
Packager : Publix Super Markets Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
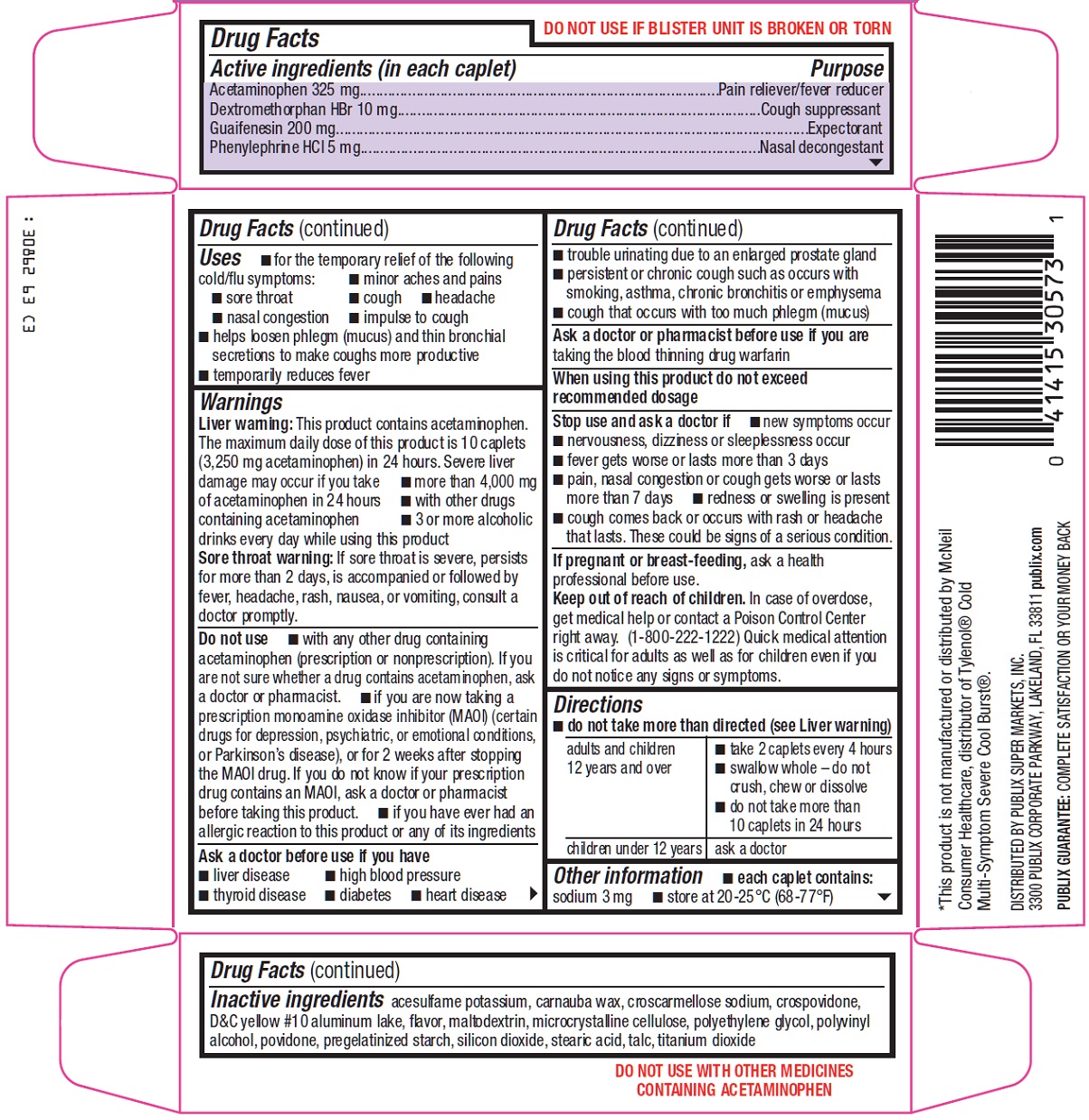
INGREDIENTS AND APPEARANCE
| severe cold Acetaminophen, Dextromethorphan Hydrobromide, Guaifenesin, Phenylephrine Hydrochloride TABLET, FILM COATED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
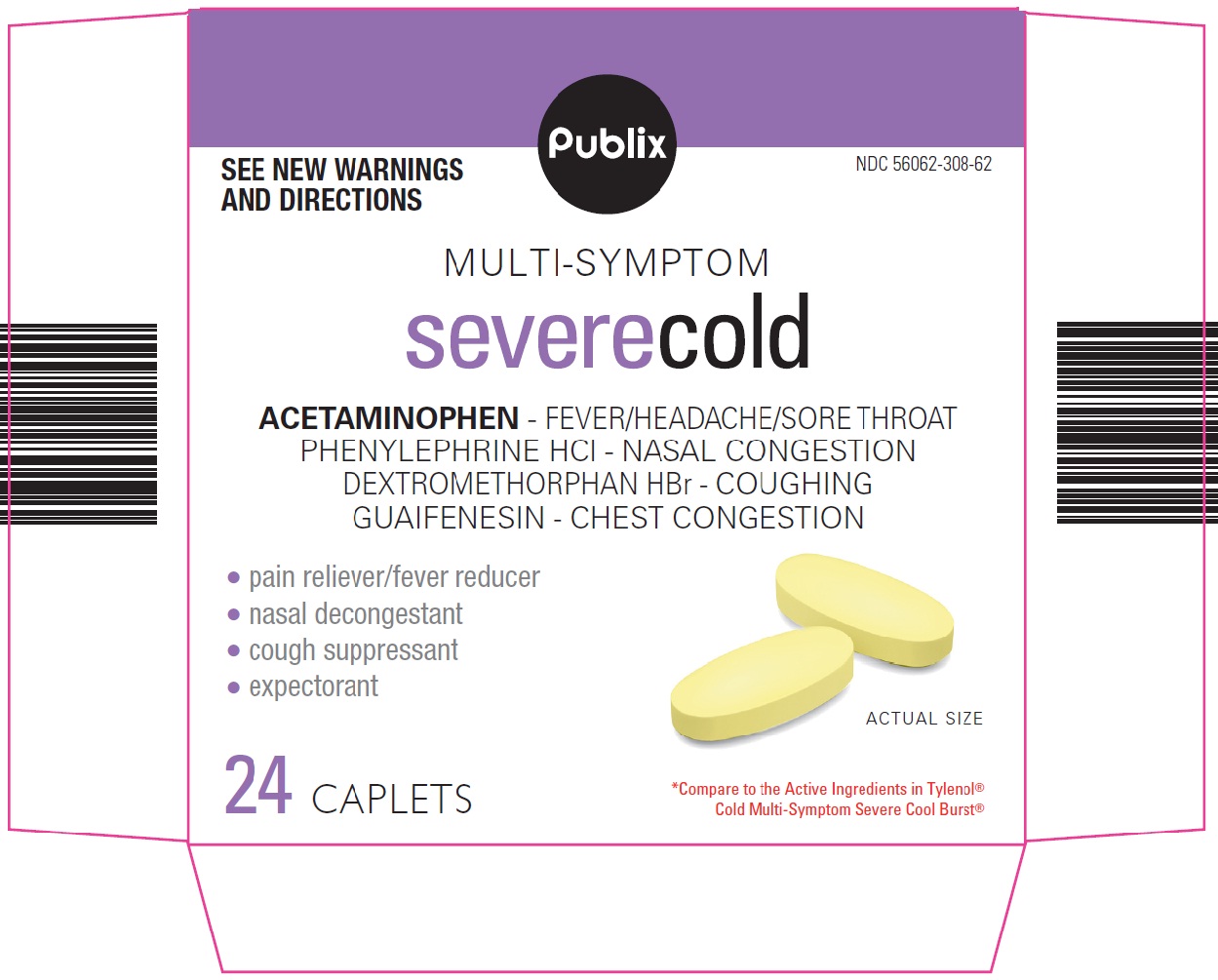
PRINCIPAL DISPLAY PANEL
SEE NEW WARNINGS AND DIRECTIONS
MULTI-SYMPTOM
severe cold
ACETAMINOPHEN – FEVER/HEADACHE/SORE THROAT
PHENYLEPHRINE HCl – NASAL CONGESTION
DEXTROMETHORPHAN HBr – COUGHING
GUAIFENESIN – CHEST CONGESTION
pain reliever/fever reducer
nasal decongestant
cough suppressant
expectorant
ACTUAL SIZE
24 CAPLETS
Compare to the Active Ingredients in Tylenol® Cold Multi-Symptom Severe Cool Burst®