NDC Code(s) : 55315-731-43
Packager : Fred's Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Miconazole 3 Combination PackMiconazole Nitrate KIT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
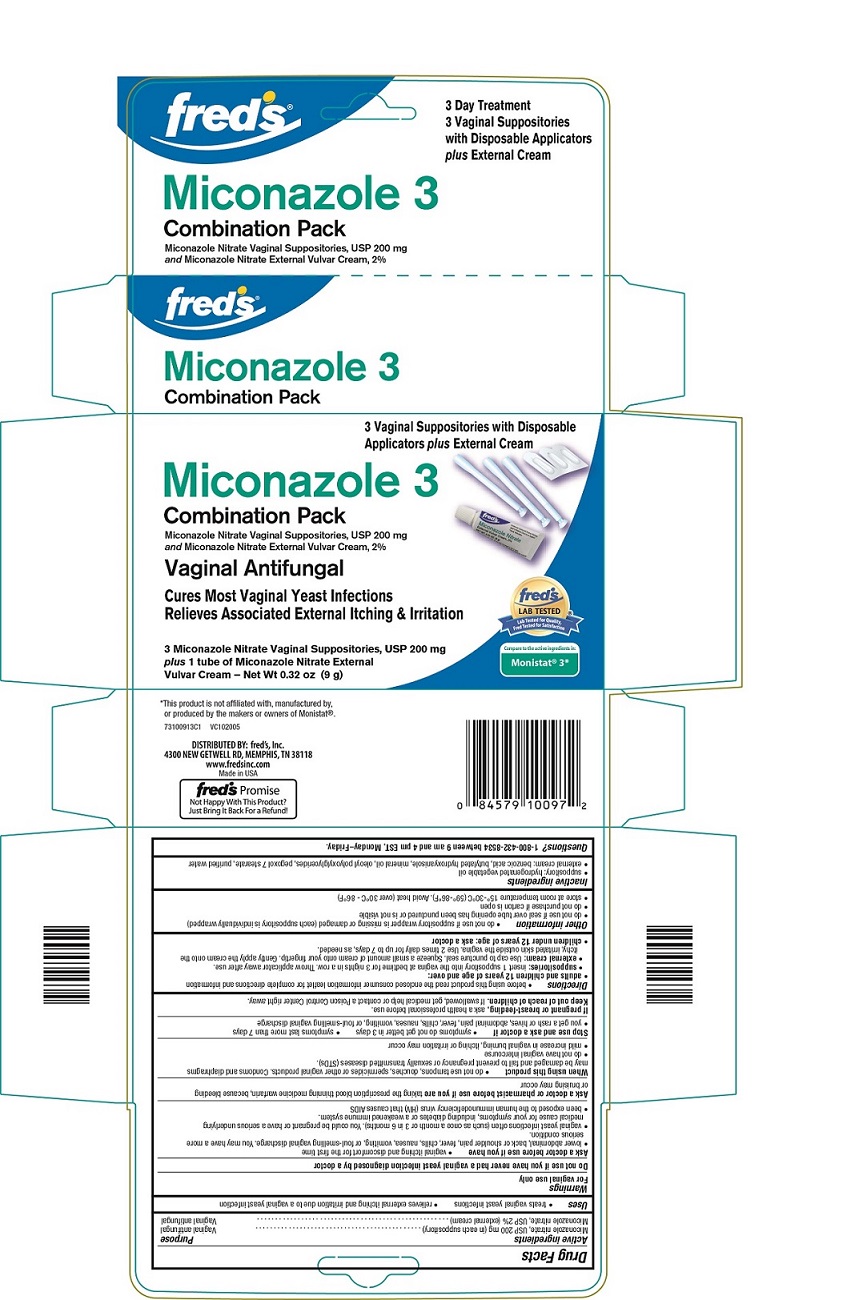
PRINCIPAL DISPLAY PANEL
fred’s®
3 Day Treatment
3 Vaginal Suppositories
with Disposable Applicators
plus External Cream
Miconazole 3
Combination Pack
Miconazole Nitrate Vaginal Suppositories, USP 200 mg
and Miconazole Nitrate External Vulvar Cream, 2%
Vaginal Antifungal
Cures Most Vaginal Yeast Infections
Relieves Associated External Itching & Irritation
3 Miconazole Nitrate Vaginal Suppositories, USP 200 mg
plus 1 tube of Miconazole Nitrate External
Vulvar Cream – Net Wt 0.32 oz (9 g)
Compare to the active ingredients in:
Monistat® 3*