NDC Code(s) : 52536-500-01, 52536-500-03, 52536-510-03, 52536-515-03, 52536-520-03, 52536-530-03
Packager : Wilshire Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CII
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Dextroamphetamine SulfateDextroamphetamine Sulfate TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Dextroamphetamine SulfateDextroamphetamine Sulfate TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Dextroamphetamine SulfateDextroamphetamine Sulfate TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Dextroamphetamine SulfateDextroamphetamine Sulfate TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Dextroamphetamine SulfateDextroamphetamine Sulfate TABLET | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| LABELER - Wilshire Pharmaceuticals, Inc.(078657245) |
PRINCIPAL DISPLAY PANEL
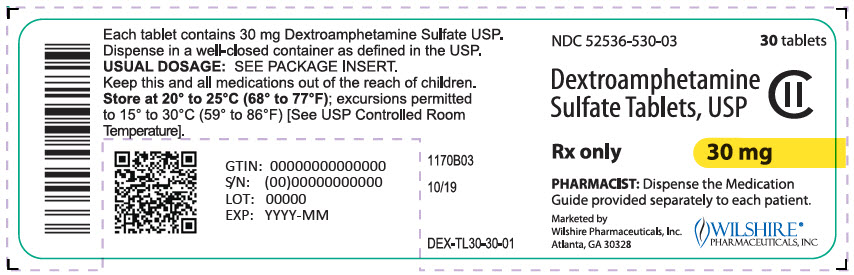
NDC 52536-500-03
30 tablets
Dextroamphetamine
Sulfate Tablets, USP
CII
Rx only
5 mg
PHARMACIST: Dispense the Medication
Guide provided separately to each patient.
Marketed by
Wilshire Pharmaceuticals, Inc.
Atlanta, GA 30328
WILSHIRE®
PHARMACEUTICALS, INC
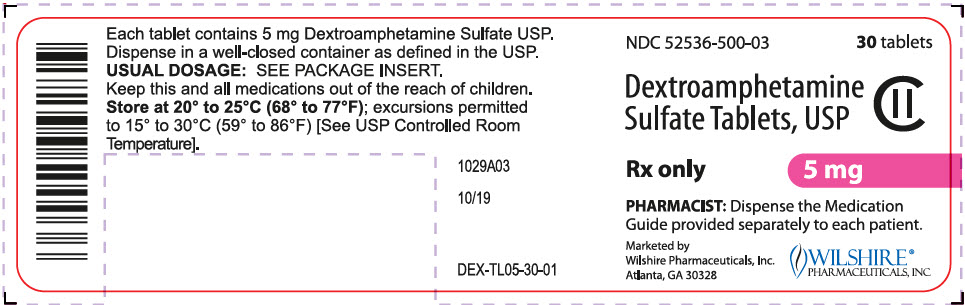
PRINCIPAL DISPLAY PANEL
NDC 52536-510-03
30 tablets
Dextroamphetamine
Sulfate Tablets, USP
CII
Rx only
10 mg
PHARMACIST: Dispense the Medication
Guide provided separately to each patient.
Marketed by
Wilshire Pharmaceuticals, Inc.
Atlanta, GA 30328
WILSHIRE®
PHARMACEUTICALS, INC
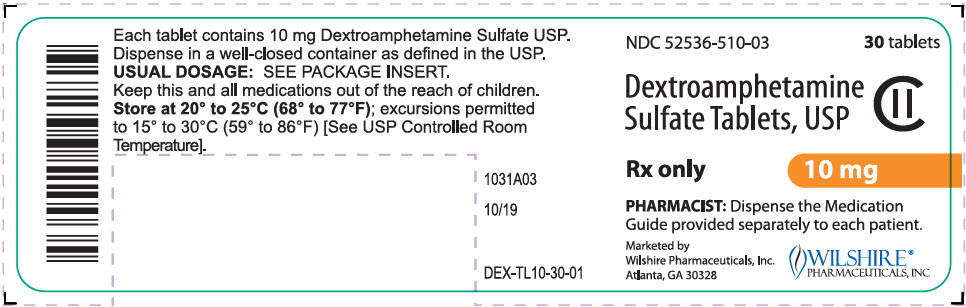
PRINCIPAL DISPLAY PANEL
NDC 52536-515-03
30 tablets
Dextroamphetamine
Sulfate Tablets, USP
CII
Rx only
15 mg
PHARMACIST: Dispense the Medication
Guide provided separately to each patient.
Marketed by
Wilshire Pharmaceuticals, Inc.
Atlanta, GA 30328
WILSHIRE®
PHARMACEUTICALS, INC
PRINCIPAL DISPLAY PANEL
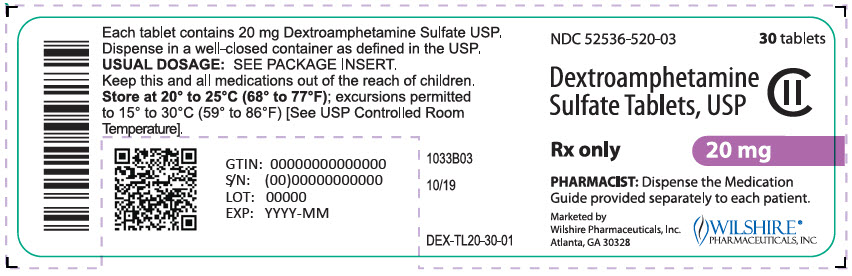
NDC 52536-520-03
30 tablets
Dextroamphetamine
Sulfate Tablets, USP
CII
Rx only
20 mg
PHARMACIST: Dispense the Medication
Guide provided separately to each patient.
Marketed by
Wilshire Pharmaceuticals, Inc.
Atlanta, GA 30328
WILSHIRE®
PHARMACEUTICALS, INC
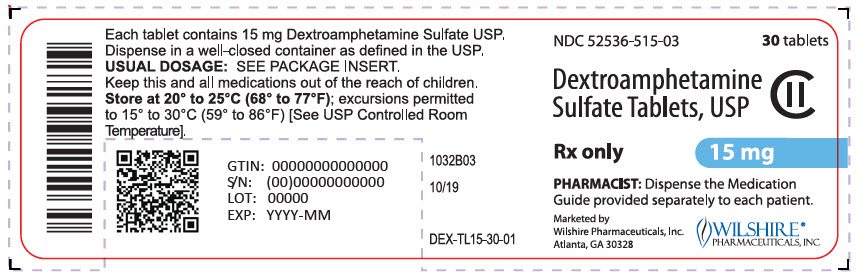
PRINCIPAL DISPLAY PANEL
NDC 52536-530-03
30 tablets
Dextroamphetamine
Sulfate Tablets, USP
CII
Rx only
30 mg
PHARMACIST: Dispense the Medication
Guide provided separately to each patient.
Marketed by
Wilshire Pharmaceuticals, Inc.
Atlanta, GA 30328
WILSHIRE®
PHARMACEUTICALS, INC