NDC Code(s) : 52268-201-01
Packager : Braintree Laboratories, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| SUTABsodium sulfate, magnesium sulfate, and potassium chloride TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Braintree Laboratories, Inc.(107904591) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Braintree Laboratories, Inc. | 617357954 | manufacture(52268-201), analysis(52268-201) | |
PRINCIPAL DISPLAY PANEL
Principal Display Panel Carton Label
NDC 52268-201-01
Please see www.sebelapharma.com for patent information.
SUTAB
(sodium sulfate, magnesium sulfate, and potassium chloride)
Tablets
1.479g/0.225g/0.188g
| NOTE TO PHARMACIST:Inform patients to REMOVE AND DISCARD the DESICCANTfrom both medication bottles before SUTAB ingestion. |
This carton contains:
2Bottles of 12 tablets each
116-ounce cup
1Patient booklet
Booklet includes:
1. Instructions for Use
2. Full Prescribing Information
3. Medication Guide
Both 12-tablet bottles are required for a complete preparation.
©2020 Braintree Laboratories Inc. All rights reserved. OCT 2023
SUT20101
Rx only
Braintree
A PART OF SEBELA PHARMACEUTICALS

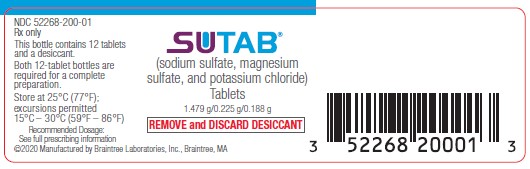
PRINCIPAL DISPLAY PANEL
Principal Display Panel Bottle Label
NDC 52268-200-01
Rx only
SUTAB
(sodium sulfate, magnesium sulfate, and potassium chloride)
Tablets
1.479g/0.225g/0.188g
| REMOVE and DISCARD DESICCANT |
This bottle contains 12 tablets and a desiccant.
Both 12-tablet bottles are required for a complete preparation.
Store at 25°C (77°F);
excursions permitted
15-30°C (59-86°F)
Recommended Dosage:
See full prescribing information
©2020 Manufactured by Braintree Laboratories, Inc. Braintree, MA