NDC Code(s) : 51672-4070-8
Packager : Taro Pharmaceuticals U.S.A., Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Cetirizine HydrochlorideCetirizine Hydrochloride SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Taro Pharmaceuticals U.S.A., Inc.(145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Taro Pharmaceutical Industries Ltd. | 600072078 | manufacture(51672-4070) | |
PRINCIPAL DISPLAY PANEL
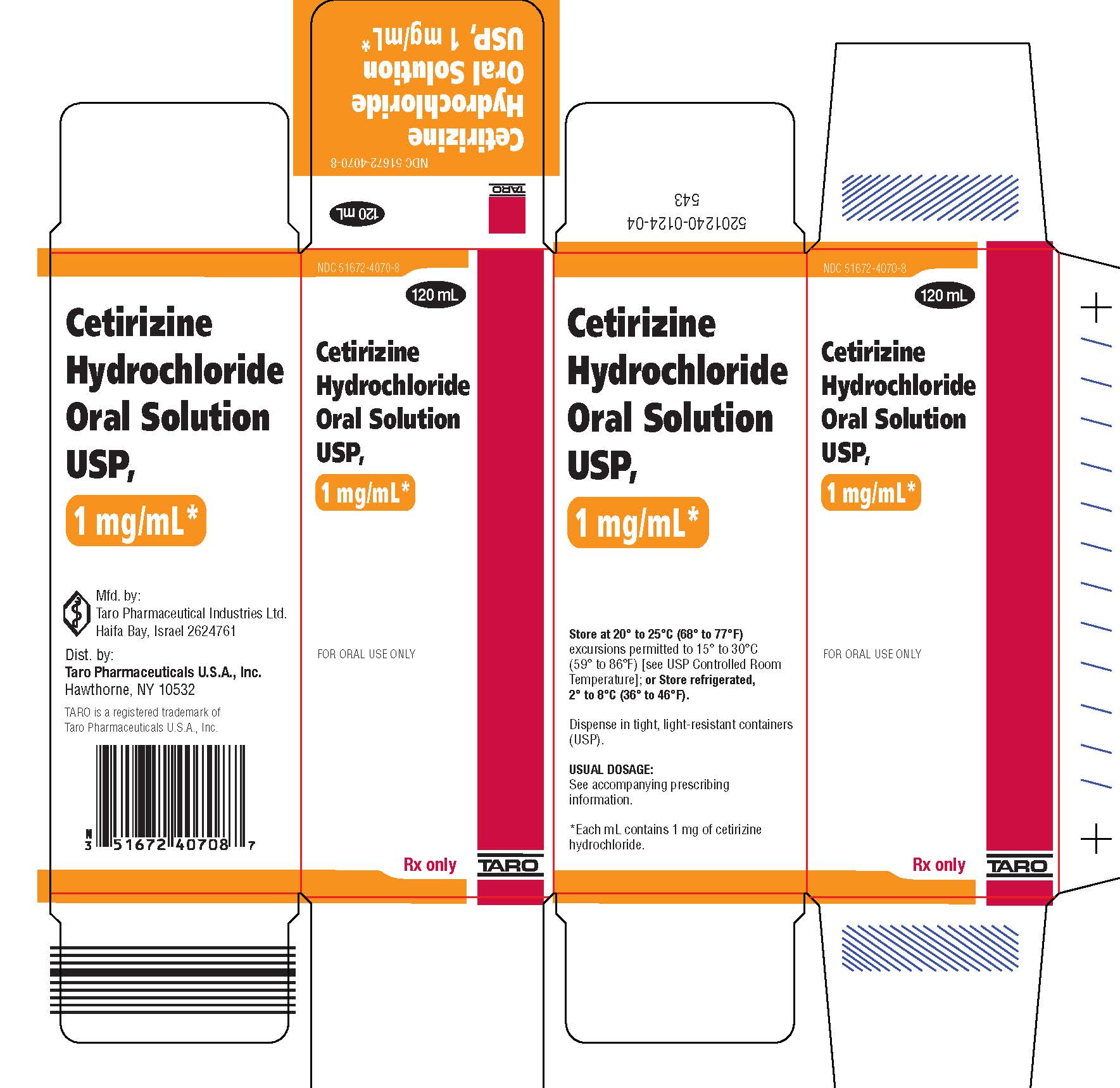 NDC 51672-4070-8
NDC 51672-4070-8
120 mL
Cetirizine
Hydrochloride
Oral Solution
USP,
1 mg/mL*
FOR ORAL USE ONLY
Rx only
TARO









