NDC Code(s) : 51407-169-30, 51407-169-01, 51407-170-01
Packager : Golden State Medical Supply, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CalcitriolCalcitriol CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CalcitriolCalcitriol CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Golden State Medical Supply, Inc.(603184490) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Golden State Medical Supply Inc. | 603184490 | relabel(51407-169, 51407-170), repack(51407-169, 51407-170) | |
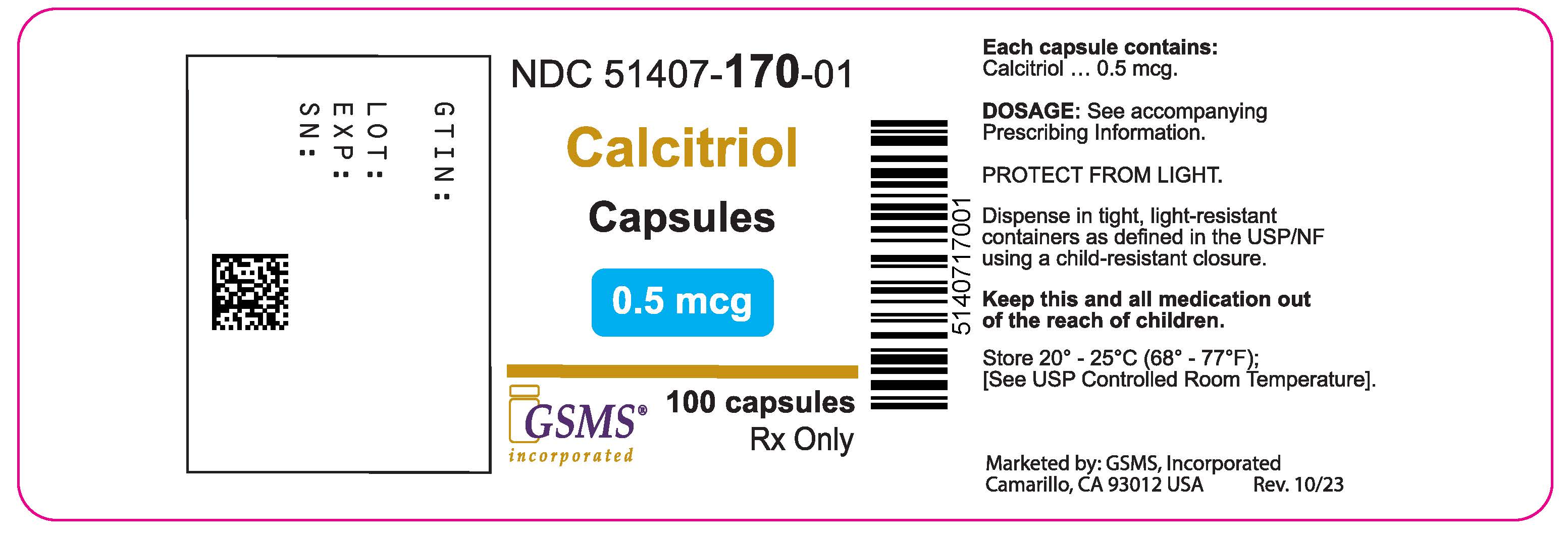
PRINCIPAL DISPLAY PANEL
Package Label - Principal Display Panel – 0.25 mcg, 100's Label
NDC 51407-169-01
Calcitriol Capsules
0.25 mcg
Rx only
100 Capsules
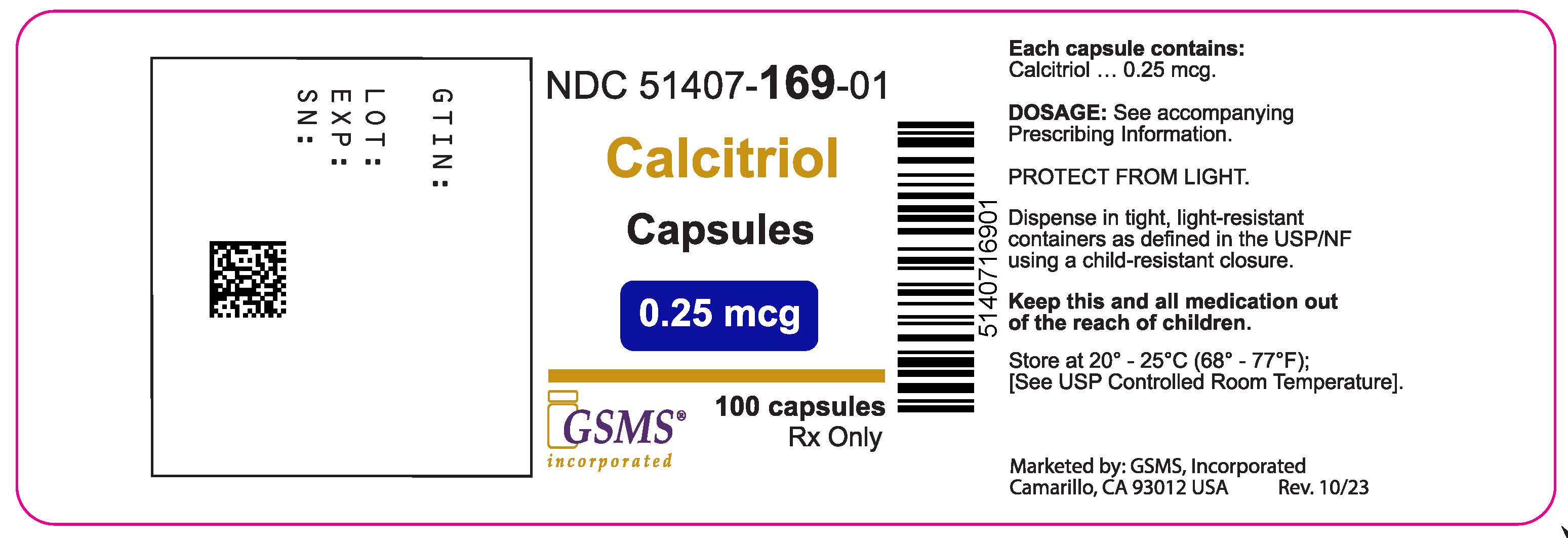
PRINCIPAL DISPLAY PANEL
Package Label - Principal Display Panel – 0.5 mcg, 100's Label
NDC 51407-170-01
Calcitriol Capsules
0.5 mcg
Rx only
100 Capsules