NDC Code(s) : 49035-929-12, 49035-929-98, 49035-929-51
Packager : Wal-Mart Stores Inc
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Allergy ReliefDiphenhydramine HCl TABLET, FILM COATED | |||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| LABELER - Wal-Mart Stores Inc(051957769) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 038154464 | pack(49035-929) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 832867837 | manufacture(49035-929), pack(49035-929) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 832867894 | manufacture(49035-929) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 868734088 | manufacture(49035-929) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| LNK International, Inc. | 967626305 | pack(49035-929) | |
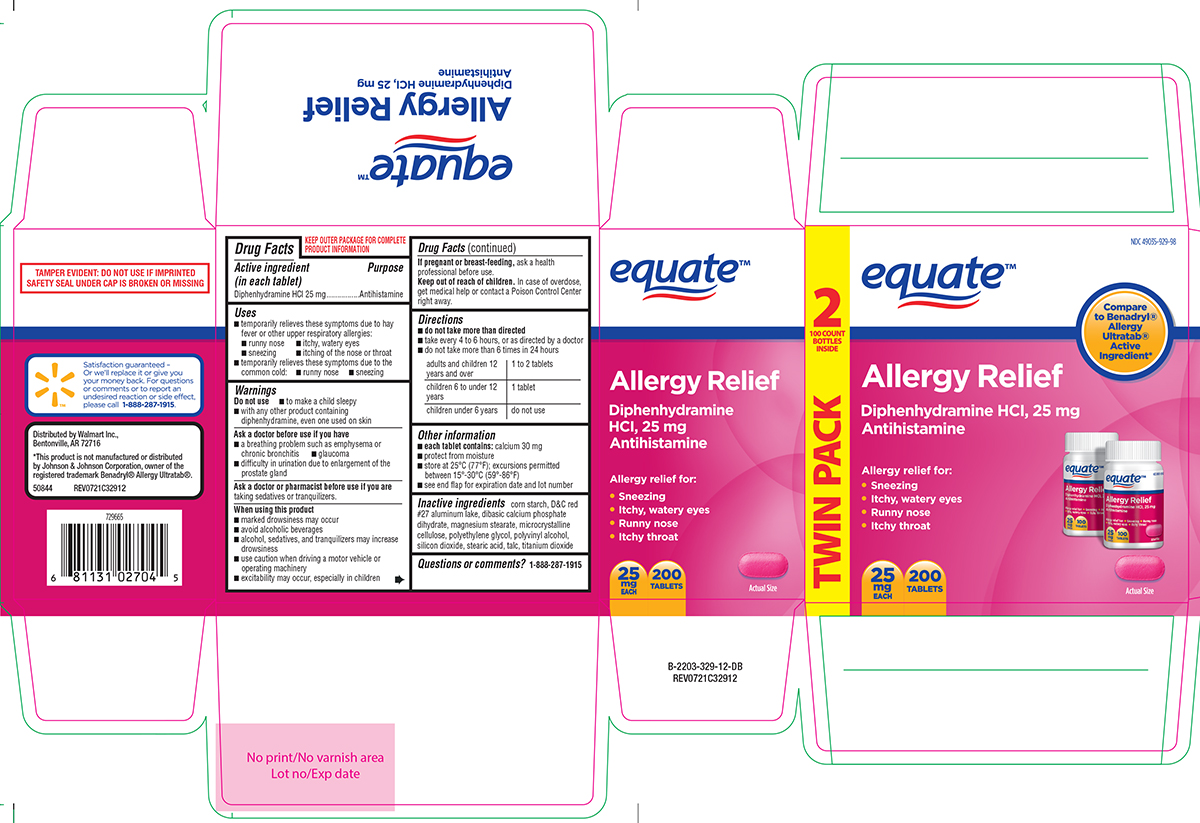
PRINCIPAL DISPLAY PANEL
equate™
NDC 49035-929-98
Compare
to Benadryl®
Allergy
Ultratab®
Active
Ingredient*
Allergy Relief
Diphenhydramine HCl, 25 mg
Antihistamine
Allergy relief for:
• Sneezing
• Itchy, watery eyes
• Runny nose
• Itchy throat
Actual Size
25
mg
EACH
200
TABLETS
TWIN PACK 2 100 COUNT BOTTLES INSIDE
TAMPER EVIDENT: DO NOT USE IF IMPRINTED
SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Distributed by Walmart Inc.,
Bentonville, AR 72716
*This product is not manufactured or distributed
by Johnson & Johnson Corporation, owner of the
registered trademark Benadryl® Allergy Ultratab®.
50844 REV0721C32912
Satisfaction guaranteed -
Or we'll replace it or give you
your money back. For questions
or comments or to report an
undesired reaction or side effect,
please call 1-888-287-1915.
 Equate 44-329
Equate 44-329