NDC Code(s) : 45567-0555-1, 45567-0555-2
Packager : Sun Pharmaceutical Industries, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Kit for the Preparation of Technetium Tc99m SestamibiTECHNETIUM TC-99M SESTAMIBI INJECTION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| LABELER - Sun Pharmaceutical Industries, Inc.(139261648) |
| REGISTRANT - Sun Pharmaceutical Industries, Inc.(139261648) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Sun Pharmaceutical Industries, Inc. | 139261648 | LABEL(45567-0555), PACK(45567-0555), ANALYSIS(45567-0555), MANUFACTURE(45567-0555) | |
PRINCIPAL DISPLAY PANEL
Kit for the Preparation of Technetium Tc99m Sestamibi Injection
1mg Cu(MIBI)4BF4 per Vial
For Intravenous Use after Reconstitution
Rx Only
Dosage: See Package Insert
Store at 20-25ºC (68-77ºF) [See USP] before and after reconstitution.
Use within 6 hours after reconstitution.
PL-000023
Rev 0.2
Mar 2020
Manufactured by:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
- Lot Exp

PRINCIPAL DISPLAY PANEL
CAUTION: RADIOACTIVE MATERIAL
See Package Insert for full prescribing information
Rx Only
Store: 20-25ºC
Use within 6 hours of reconstitution
Technetium Tc99m Sestamibi Injection
Contents
1mg Tetrakis (2-methoxy isobutyl isonitrile)
Copper (I) tetrafluoroborate [Cu(MIBI)4BF4]
2.6mg Sodium Citrate dihydrate
1mg L-Cysteine hydrochloride monohydrate
20mg Mannitol
0.025mg minimum stannous chloride dihydrate
0.075mg stannous chloride dihydrate
0.086mg maximum tin chloride (stannous and stannic) dihydrate
Sodium Pertechnetate Tc99m Injection
____________________________________________________
MBq (mCi) Tc99m/mL
____________________________________________________
Volume mL
____________________________________________________
Date / Time prepared Expiration Time
______________________
Lot No.
PL-000024
Rev 0.2
Mar 2020
Manufactured by:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821

PRINCIPAL DISPLAY PANEL
Kit for the
Preparation of Technetium Tc99m
Sestamibi Injection
Kit for the
Preparation of Technetium Tc99m
Sestamibi Injection
1mg Cu(MIBI)4BF4 per Vial
For Intravenous Use after
Reconstitution
Rx Only
Nonradioactive • Diagnostic Agent • Multidose
Sterile • Non-Pyrogenic
Vial contents are sealed under Nitrogen
at time of manufacture. The pH of
the reconstituted product is 5.5
Dosage: See Package Insert
Manufactured by:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
For Customer Service call: 1-800-221-7554
Kit for the
Preparation of Technetium Tc99m
Sestamibi Injection
for:
Myocardial or Breast Imaging
Rx Only
Each sterile, lyophilized vial contains: 1mg Tetrakis
(2-methoxy isobutyl isonitrile) Copper (I) tetrafluoroborate
[Cu(MIBI)4BF4]; 2.6mg Sodium Citrate dihydrate; 1mg
L-Cysteine Hydrochloride monohydrate; 20mg Mannitol;
0.025mg minimum stannous chloride dihydrate; 0.075mg stannous
chloride dihydrate; 0.086mg maximum tin chloride (stannous
and stannic) dihydrate. pH of reconstituted product is 5.5
CONTAINS NO PRESERVATIVE
Reconstitute with oxidant-free Tc99m
Before reconstitution and after labeling
with oxidant-free Technetium Tc 99m,
store at 20-25°C (68-77°F) [See USP]
After labeling store in a suitable lead shield
and use within 6 hours (see insert).
Contents: 1 package insert, 12 radiation
labels and 5 reaction vials
Preparation: See Package Insert for complete
information, use and directions
Kit for the
Preparation of
Technetium Tc99m
Sestamibi
Injection
IMPORTANT:
Read Package Insert for full
information on preparation,
use and indications.
WARNING:
Radiopharmaceuticals should
be used by persons who are
qualified by specific training
in the safe use and handling
of radionuclides and whose
experience and training have
been approved by the
appropriate governmental
agency.
PL-000025
Rev 0.3
Mar 2020

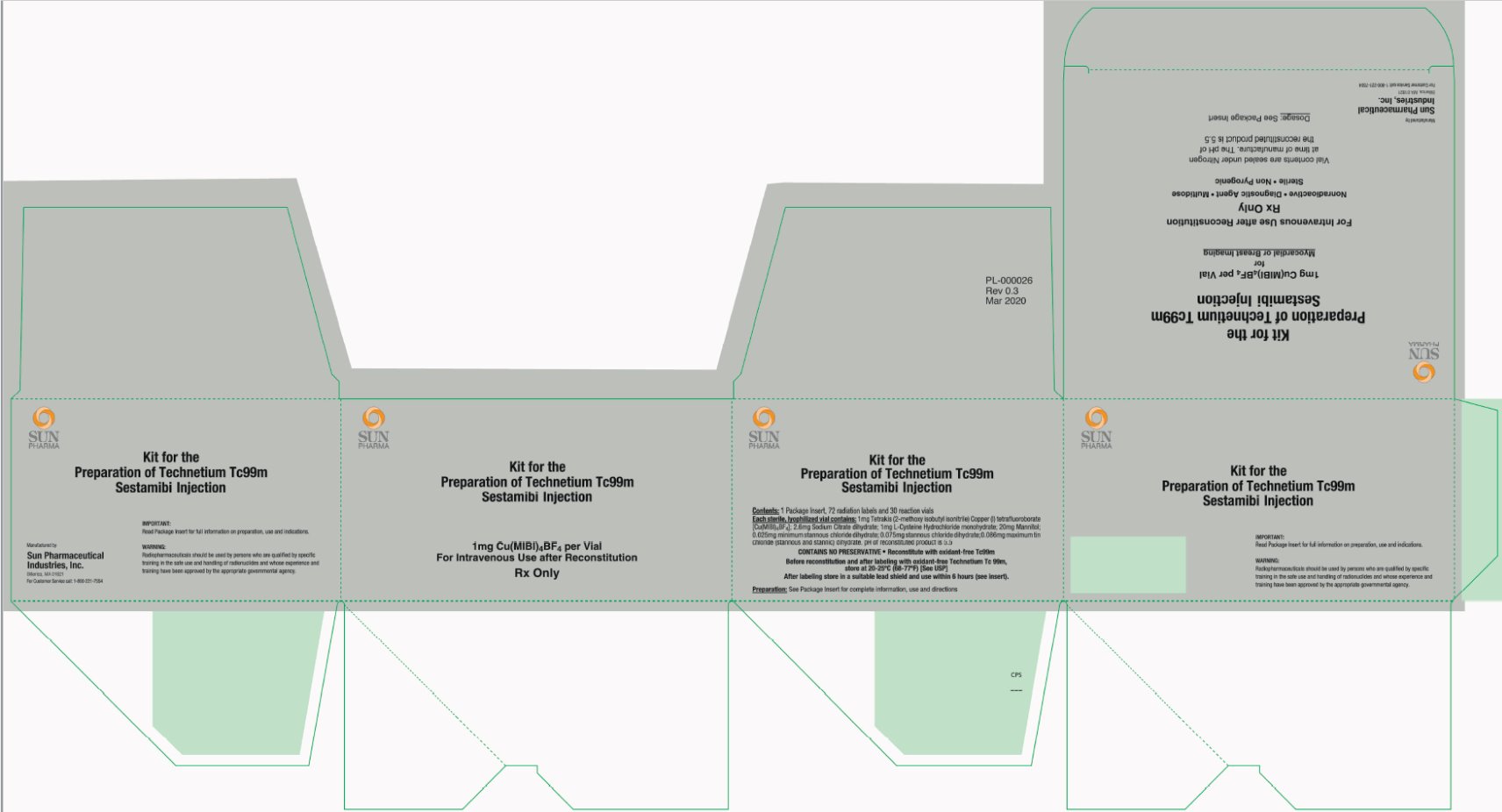
PRINCIPAL DISPLAY PANEL
Kit for the
Preparation of Technetium Tc99m
Sestamibi Injection
1mg Cu(MIBI)4BF4 per Vial
for
Myocardial or Breast Imaging
For Intravenous Use after Reconstitution
Rx Only
Nonradioactive • Diagnostic Agent • Multidose
Sterile • Non-Pyrogenic
Vial contents are sealed under Nitrogen
at time of manufacture. The pH of
the reconstituted product is 5.5
Dosage: See Package Insert
Manufactured by:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
For Customer Service call: 1-800-221-7554
Kit for the
Preparation of Technetium Tc99m
Sestamibi Injection
Contents: 1 Package Insert, 72 radiation labels and 30 reaction vials
Each sterile, lyophilized vial contains: 1mg Tetrakis (2-methoxy isobutyl isonitrile) Copper (I) tetrafluoroborate [Cu(MIBI)4BF4]; 2.6mg Sodium Citrate dihydrate; 1mg L-Cysteine Hydrochloride monohydrate; 20mg Mannitol; 0.025mg minimum stannous chloride dihydrate; 0.075mg stannous chloride dihydrate; 0.086mg maximum tin chloride (stannous and stannic) dihydrate. pH of reconstituted product is 5.5
CONTAINS NO PRESERVATIVE • Reconstitute with oxidant-free Tc99m
Before reconstitution and after labeling with oxidant-free Technetium Tc 99m,
store at 20-25°C (68-77°F) [See USP]
After labeling store in a suitable lead shield and use within 6 hours (see insert).
Preparation: See Package Insert for complete information, use and directions
Kit for the
Preparation of Technetium Tc99m
Sestamibi Injection
1mg Cu(MIBI)4BF4 per Vial
For Intravenous Use after Reconstitution
Rx Only
Kit for the
Preparation of Technetium Tc99m
Sestamibi Injection
IMPORTANT:
Read Package Insert for full information on preparation, use and indications.
WARNING:
Radiopharmaceuticals should be used by persons who are qualified by specific
training in the safe use and handling of radionuclides and whose experience and
training have been approved by the appropriate governmental agency.
Manufactured by:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
For Customer Service call: 1-800-221-7554
PL-000026
Rev 0.3
Mar 2020