NDC Code(s) : 42023-159-01, 42023-159-25, 42023-168-01
Packager : ENDO USA, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Adrenalinepinephrine INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Adrenalinepinephrine INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - ENDO USA, Inc.(119185057) |
PRINCIPAL DISPLAY PANEL
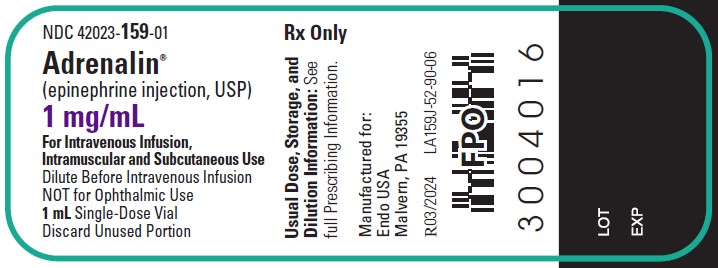
1 mL Vial Label
NDC 42023-159-01
Rx Only
Adrenalin®
(epinephrine injection, USP)
1 mg/mL
For Intravenous Infusion, Intramuscular and Subcutaneous Use
NOT for Ophthalmic Use
1 mL Single Dose Vial
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 30 mL Vial Label
NDC 42023-168-01
Rx Only
Adrenalin®
(epinephrine injection, USP)
30 mg/30 mL
(1 mg/mL)
For Intravenous Infusion, Intramuscular and S ubcutaneous Use
NOT for Ophthalmic Use
30 mL Multiple Dose Vial

PRINCIPAL DISPLAY PANEL
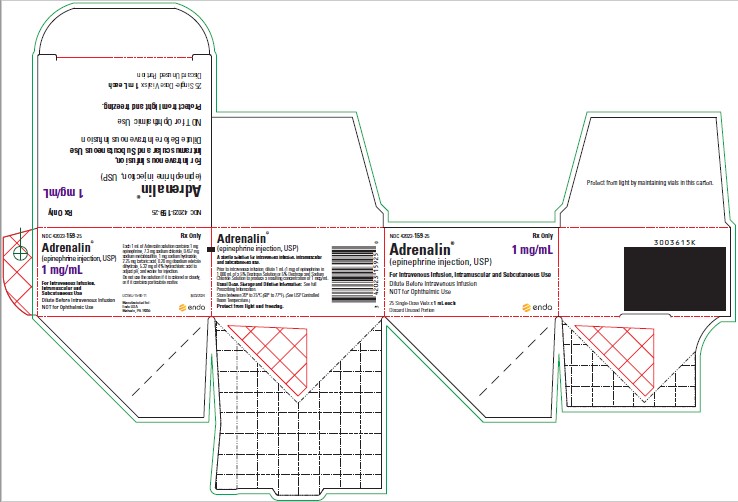
1 mL Carton
NDC 42023-159-25
Rx Only
Adrenalin®
(epinephrine injection, USP)
1 mg/mL
For Intravenous Infusion, Intramuscular and Subcutaneous Use
NOT for Ophthalmic Use
PRINCIPAL DISPLAY PANEL
30 mL Carton
NDC 42023-168-01
Rx Only
Adrenalin®
(epinephrine injection, USP)
30 mg/mL
For Intravenous Infusion, Intramuscular and Subcutaneous Use
NOT for Ophthalmic Use










