NDC Code(s) : 41616-882-40, 41616-882-44
Packager : Sun Pharma Global Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| NICARdipine Hydrochloride NICARdipine Hydrochloride INJECTION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
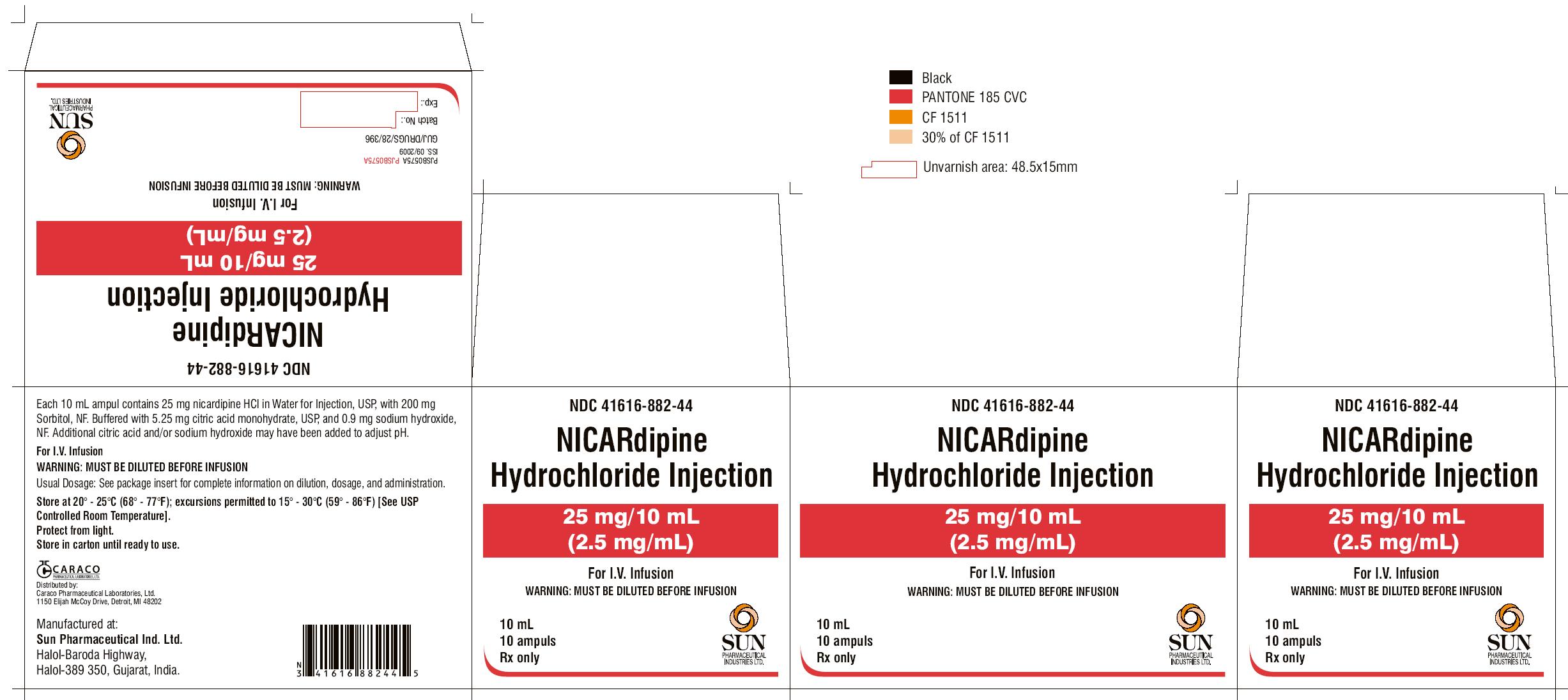
PRINCIPAL DISPLAY PANEL
NDC 41616-882-40
NICARdipine Hydrochloride Injection
25 mg/10 mL
(2.5 mg/mL)
For I.V. Infusion
10 mL ampul
Rx only
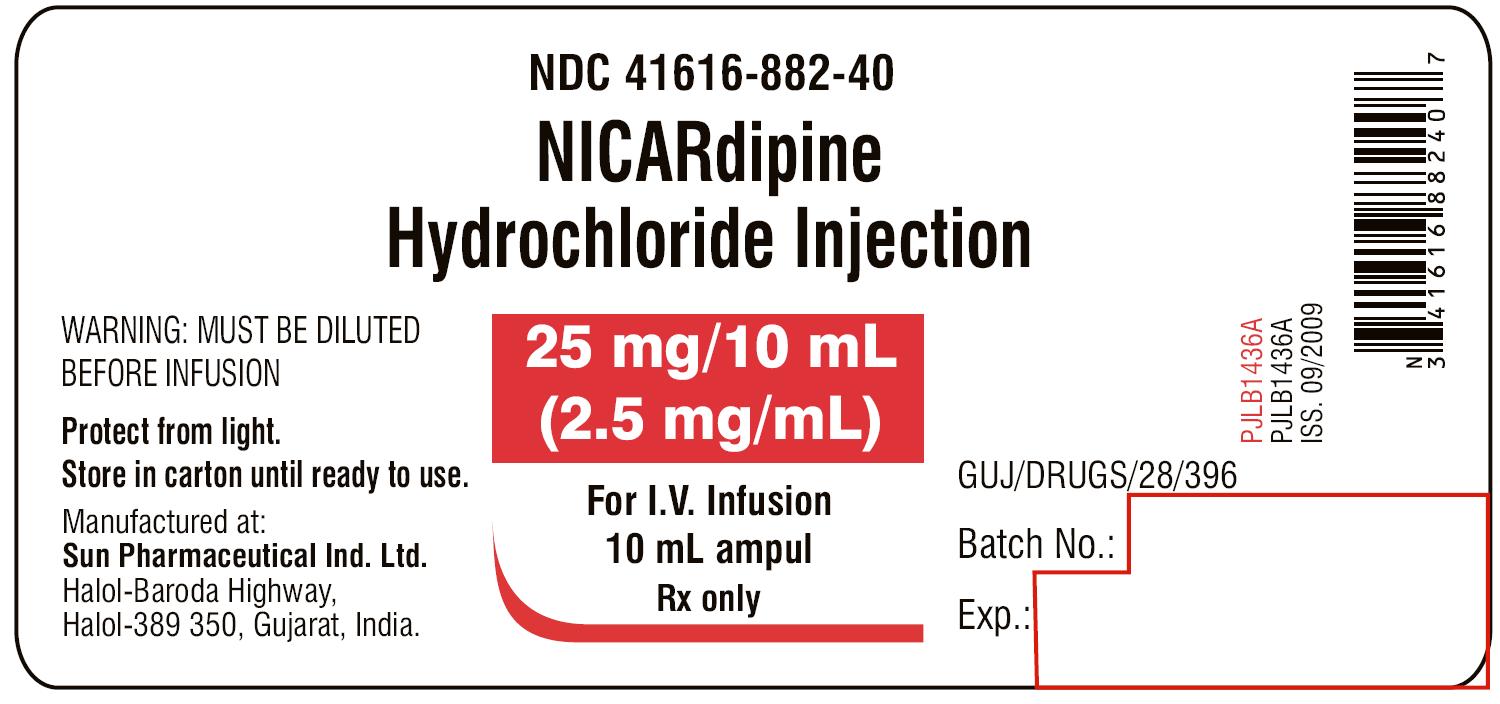
PRINCIPAL DISPLAY PANEL
NDC 41616-882-40
NICARdipine Hydrochloride Injection
25 mg/10 mL
(2.5 mg/mL)
For I.V. Infusion
WARNING: MUST BE DILUTED BEFORE INFUSION
10 mL ampul
Rx only
SUN PHARMACEUTICAL INDUSTRIES LTD.
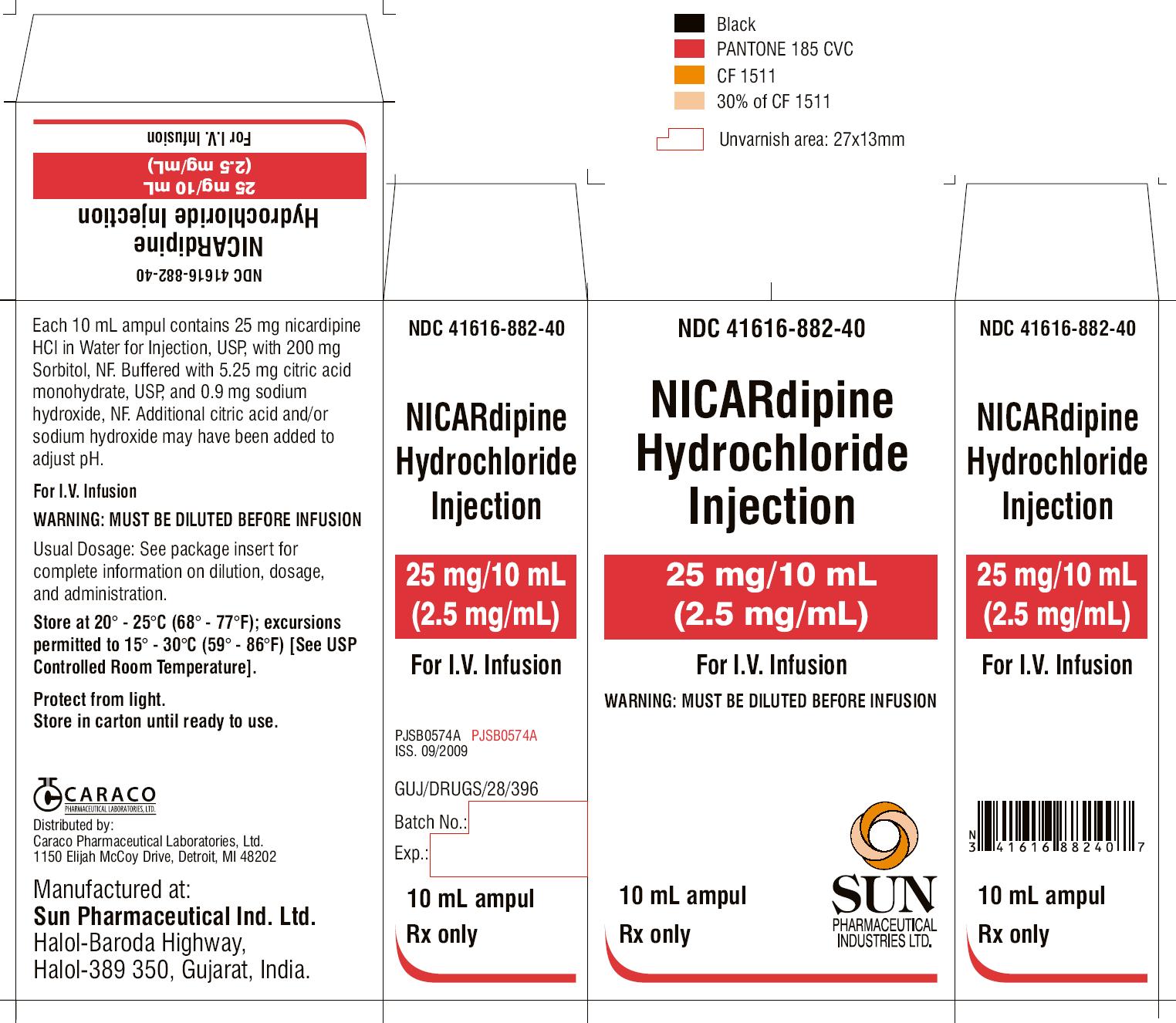
PRINCIPAL DISPLAY PANEL
NDC 41616-882-44
NICARdipine Hydrochloride Injection
25 mg/10 mL
(2.5 mg/mL)
For I.V. Infusion
WARNING: MUST BE DILUTED BEFORE INFUSION
10 mL
10 ampuls
Rx only
SUN PHARMACEUTICAL INDUSTRIES LTD.