NDC Code(s) : 41163-121-40
Packager : SuperValu (Equaline)
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| IbuprofenIbuprofen CAPSULE, LIQUID FILLED | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Ibuprofen Capsules, 200 mg
Pain reliever/ fever reducer (NSAID)
40 Softgels**
(liquid filled capsules**)
Compare to Advil® active ingredient †
SEE NEW WARNINGS INFORMATION
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
Distributed by SUPERVALU INC.
EDEN PRAIRIE, MN 55344 USA
PRODUCT OF USA
Contact us at 1-877-932-7948 or www.supervalu-ourownbrands.com
PRINCIPAL DISPLAY PANEL
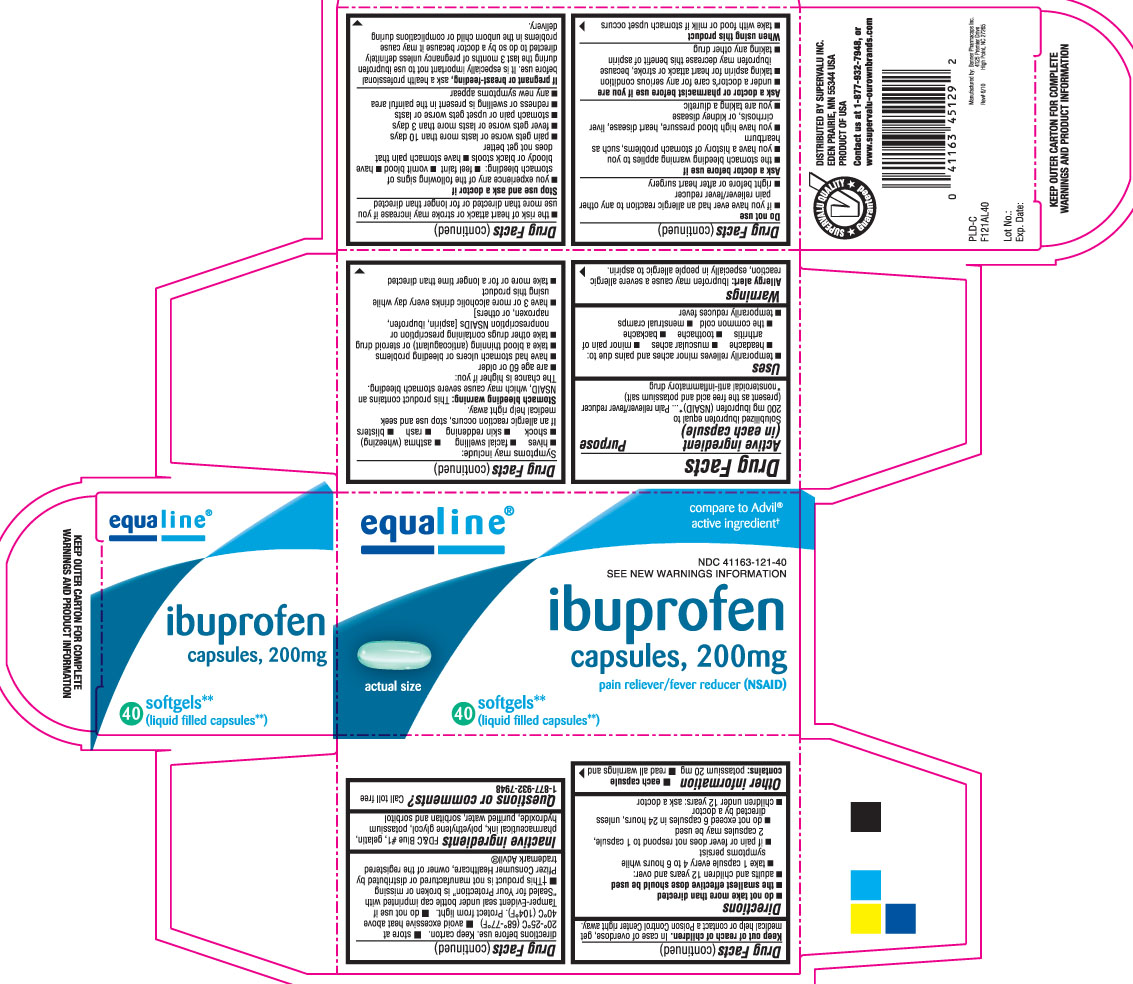 Ibuprofen capsules 200 mg
Ibuprofen capsules 200 mg









