NDC Code(s) : 24987-377-10, 24987-378-10, 24987-379-34, 24987-382-37, 24987-434-00, 24987-435-00, 24987-412-00, 24987-413-00
Packager : Covis Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| FORTAZceftazidime INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FORTAZceftazidime INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FORTAZceftazidime INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FORTAZceftazidime INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FORTAZceftazidime INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FORTAZceftazidime INJECTION, POWDER, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FORTAZceftazidime sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| FORTAZceftazidime sodium INJECTION, SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
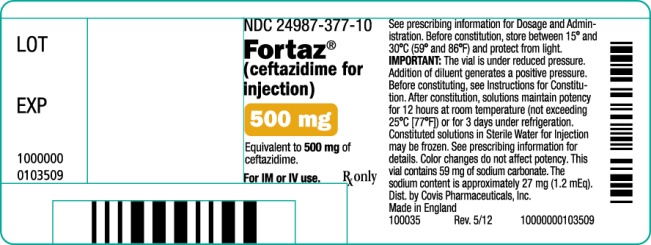 500 mg Vial
500 mg Vial
NDC 24987-377-10
Fortaz®
(ceftazidime for injection)
500 mg
Equivalent to 500 mg of ceftazidime.
For IM or IV use.
Rx only
See prescribing information for Dosage and Administration. Before constitution, store between 15o and 30oC (59o and 86oF) and protect from light.
IMPORTANT: The vial is under reduced pressure. Addition of diluent generates a positive pressure. Before constituting, see Instructions for Constitution. After constitution, solutions maintain potency for 12 hours at room temperature (not exceeding 25oC [77oF]) or for 3 days under refrigeration. Constituted solutions in Sterile Water for Injection may be frozen. See prescribing information for details. Color changes do not affect potency. This vial contains 59 mg of sodium carbonate. The sodium content is approximately 27 mg (1.2 mEq).
Dist. by Covis Pharmaceuticals, Inc.
Made in England
100035
Rev. 5/12
10000000103509
LOT
EXP
10000000103509
PRINCIPAL DISPLAY PANEL
 1 gram Vial
1 gram Vial
NDC 24987-378-10
Fortaz®
(ceftazidime for injection)
1 g
Equivalent to 1 g of ceftazidime.
For IM or IV use.
Rx only
See prescribing information for Dosage and Administration. Before constitution, store between 15o and 30oC (59o and 86oF) and protect from light.
IMPORTANT: The vial is under reduced pressure. Addition of diluent generates a positive pressure. Before constituting, see Instructions for Constitution. After constitution, solutions maintain potency for 12 hours at room temperature (not exceeding 25oC [77oF]) or for 3 days under refrigeration. Constituted solutions in Sterile Water for Injection may be frozen. See prescribing information for details. Color changes do not affect potency. This vial contains 118 mg of sodium carbonate. The sodium content is approximately 54 mg (2.3 mEq).
Dist. by Covis Pharmaceuticals, Inc.
Made in England
Rev. 5/12
100037
10000000103726
LOT
EXP
10000000103726
PRINCIPAL DISPLAY PANEL
 2 gram Vial
2 gram Vial
NDC 24987-379-34
Fortaz®
(ceftazidime for injection)
2 g
Equivalent to 2 g of ceftazidime.
For IV use.
Rx only
See prescribing information for Dosage and Administration. Before constitution, store between 15o and 30oC (59o and 86oF) and protect from light.
IMPORTANT: The vial is under reduced pressure. Addition of diluent generates a positive pressure. Before constituting, see Instructions for Constitution. To prepare IV solution, add 10 mL of Sterile Water for Injection. After constitution, solutions maintain potency for 12 hours at room temperature (not exceeding 25oC [77oF]) or for 3 days under refrigeration. Constituted solutions in Sterile Water for Injection may be frozen. See prescribing information for details. Color changes do not affect potency. This vial contains 236 mg of sodium carbonate. The sodium content is approximately 108 mg (4.7 mEq).
Dist. by Covis Pharmaceuticals, Inc.
Made in England
100031
Rev. 5/12
10000000103587
LOT
EXP
10000000103587
PRINCIPAL DISPLAY PANEL
 6 gram Pharmacy Bulk Package
6 gram Pharmacy Bulk Package
NDC 24987-382-37
Fortaz®
(ceftazidime for injection)
6 g
Equivalent to 6 g of ceftazidime.
Pharmacy Bulk Package
Rx only
See package insert for Dosage and Administration.
Before constitution, store between 15o and 30oC (59o and 86oF) and protect from light.
IMPORTANT: The vial is under reduced pressure. Addition of diluent generates a positive pressure. Before constituting, see Instructions for Constitution.
To prepare solution, add 26 mL of Sterile Water for Injection. Shake well to dissolve. The constituted solution occupies a volume of about 31.5 mL and contains approximately 1 g of ceftazidime activity per 5 mL.
After constitution, solutions maintain potency for 12 hours at room temperature (not exceeding 25oC [77oF]) or for 3 days under refrigeration. Color changes do not affect potency. This vial contains 709 mg of sodium carbonate. The sodium content is approximately 54 mg (2.3 mEq) per gram of ceftazidime.
Dist. by Covis Pharmaceuticals, Inc.
Cary, NC 27511
Made in England
10000000103768
100039
Rev. 5/12
LOT
EXP
PRINCIPAL DISPLAY PANEL
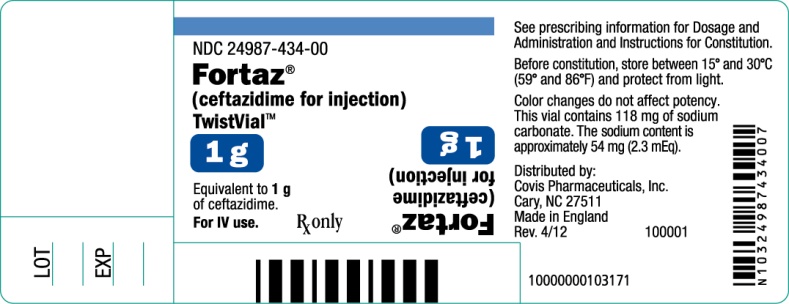 1 gram TwistVialTM Vial
1 gram TwistVialTM Vial
NDC 24987-434-00
Fortaz®
(ceftazidime for injection)
TwistVialTM
1 g
Equivalent to 1 g of ceftazidime.
For IV use.
Rx only
See prescribing information for Dosage and Administration and Instructions for Constitution.
Before constitution, store between 15o and 30oC (59o and 86oF) and protect from light.
Color changes do not affect potency. This vial contains 118 mg of sodium carbonate. The sodium content is approximately 54 mg (2.3 mEq).
Distributed by:
Covis Pharmaceuticals, Inc.
Cary, NC 27511
Made in England
Rev. 4/12
100001
10000000103171
LOT
EXP
PRINCIPAL DISPLAY PANEL
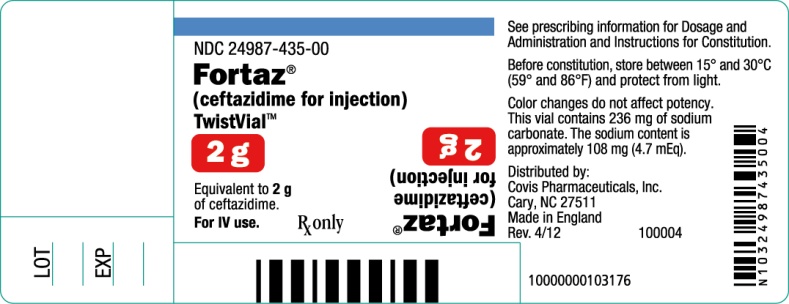 2 gram TwistVialTM Vial
2 gram TwistVialTM Vial
NDC 24987-435-00
Fortaz®
(ceftazidime for injection)
TwistVialTM
2 g
Equivalent to 2 g of ceftazidime.
For IV use.
Rx only
See prescribing information for Dosage and Administration and Instructions for Constitution.
Before constitution, store between 15o and 30oC (59o and 86oF) and protect from light.
Color changes do not affect potency. This vial contains 236 mg of sodium carbonate. The sodium content is approximately 108 mg (4.7 mEq).
Distributed by:
Covis Pharmaceuticals, Inc.
Cary, NC 27511
Made in England
Rev. 4/12
100004
10000000103176
LOT
EXP
PRINCIPAL DISPLAY PANEL
 1 gram Single-Dose Container Label
1 gram Single-Dose Container Label
Fortaz® (ceftazidime injection)
12 - 50 mL Single-Dose Containers ISO-osmotic
Do Not Store Above -20oC. Do Not Refreeze.
1 g
Covis Pharmaceuticals, Inc.
Cary, NC 27511
COVIS
NDC 24987-412-00
Code 2G3535
(01) 20324987412002
GALAXY Container
Each 50 mL contains: ceftazidime sodium equivalent to 1 g of ceftazidime with approx. 2.2 g of Dextrose Hydrous, USP added to adjust osmolality. pH adjusted with sodium hydroxide and may have been adjusted with hydrochloric acid. pH 5.0 to 7.5.
Dosage: Intravenously as directed by a physician. See prescribing information.
Cautions: Do not add supplementary medication. Must not be used in series connections. Check for minute leaks by squeezing thawed bag firmly. If leaks are found, discard bag as sterility may be impaired. Do not use unless solution is clear.
Rx only
Sterile, Nonpyrogenic
Do not store above -20oC. Thaw at room temperature (25oC) or under refrigeration (5oC). DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION. Thawed solution is stable for 3 days under refrigeration or 8 hours at room temperature. Do not refreeze.
Fortaz is a registered trademark of GlaxoSmithKline
GALAXY is a trademark of Baxter International Inc.
Distributed by Covis Pharmaceuticals, Inc., Cary, NC 27511
Manufactured by Baxter Healthcare Corporation, Deerfield, IL 60015 USA
PL 2040 Plastic
Made in England
100012
07-04-68-558
Rev. 5/12
PRINCIPAL DISPLAY PANEL
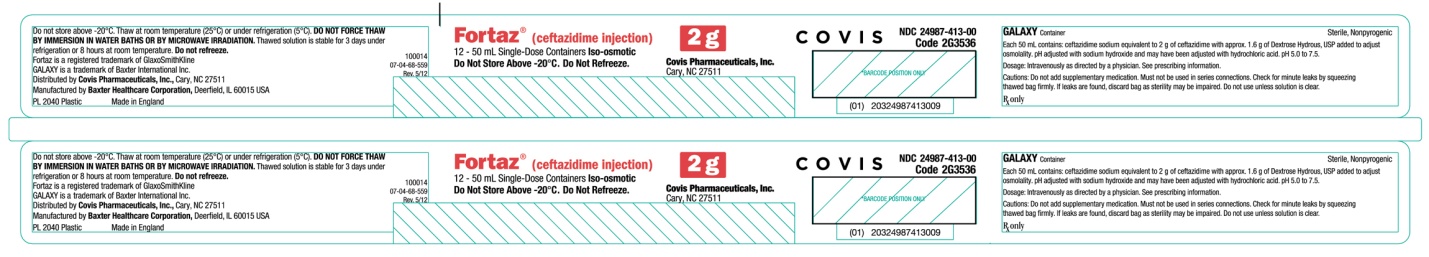 2 gram Single-Dose Container Label
2 gram Single-Dose Container Label
Fortaz® (ceftazidime injection)
12 - 50 mL Single-Dose Containers ISO-osmotic
Do Not Store Above -20oC. Do Not Refreeze.
2 g
Covis Pharmaceuticals, Inc.
Cary, NC 27511
COVIS
NDC 24987-413-00
Code 2G3536
(01) 20324987413009
GALAXY Container
Each 50 mL contains: ceftazidime sodium equivalent to 2 g of ceftazidime with approx. 1.6 g of Dextrose Hydrous, USP added to adjust osmolality. pH adjusted with sodium hydroxide and may have been adjusted with hydrochloric acid. pH 5.0 to 7.5.
Dosage: Intravenously as directed by a physician. See prescribing information.
Cautions: Do not add supplementary medication. Must not be used in series connections. Check for minute leaks by squeezing thawed bag firmly. If leaks are found, discard bag as sterility may be impaired. Do not use unless solution is clear.
Rx only
Sterile, Nonpyrogenic
Do not store above -20oC. Thaw at room temperature (25oC) or under refrigeration (5oC). DO NOT FORCE THAW BY IMMERSION IN WATER BATHS OR BY MICROWAVE IRRADIATION. Thawed solution is stable for 3 days under refrigeration or 8 hours at room temperature. Do not refreeze.
Fortaz is a registered trademark of GlaxoSmithKline
GALAXY is a trademark of Baxter International Inc.
Distributed by Covis Pharmaceuticals, Inc., Cary, NC 27511
Manufactured by Baxter Healthcare Corporation, Deerfield, IL 60015 USA
PL 2040 Plastic
Made in England
100014
07-04-68-559
Rev. 5/12









