NDC Code(s) : 17156-022-05
Packager : Medi-Physics Inc. dba GE Healthcare.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CERETECTECHNETIUM TC-99M EXAMETAZIME INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Medi-Physics Inc. dba GE Healthcare.(095263729) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| GE Healthcare AS | 515048908 | MANUFACTURE(17156-022), API MANUFACTURE(17156-022) | |
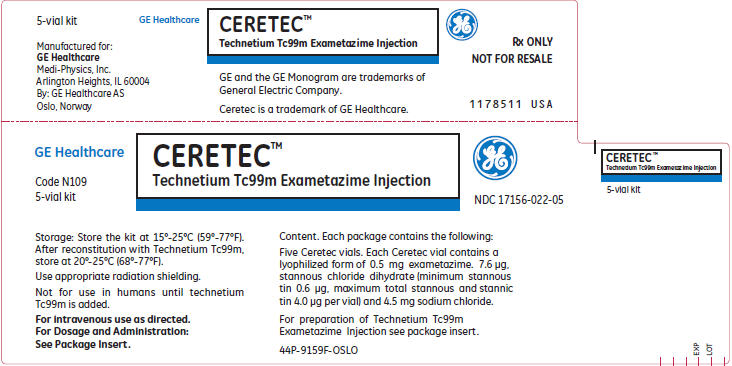
PRINCIPAL DISPLAY PANEL
GE Healthcare
CERETEC™
Technetium Tc99m Exametazime Injection
Code N109
5-vial kit
NDC 17156-022-05
Storage: Store the kit at 15°-25°C (59°-77°F).
After reconstitution with Technetium Tc99m,
store at 20°-25°C (68°-77°F).
Use appropriate radiation shielding.
Not for use in humans until technetium
Tc99m is added.
For intravenous use as directed.
For Dosage and Administration:
See Package Insert.
Content. Each package contains the following:
Five Ceretec vials. Each Ceretec vial contains a
lyophilized form of 0.5 mg exametazime. 7.6 μg,
stannous chloride dihydrate (minimum stannous
tin 0.6 μg, maximum total stannous and stannic
tin 4.0 μg per vial) and 4.5 mg sodium chloride.
For preparation of Technetium Tc99m
Exametazime Injection see package insert.
44P-9159F-OSLO